Recovery method of hydrometallurgy iron slag
A recovery method, hydrometallurgy technology, applied in the field of hydrometallurgy iron slag recovery, to achieve good application prospects, low equipment requirements, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
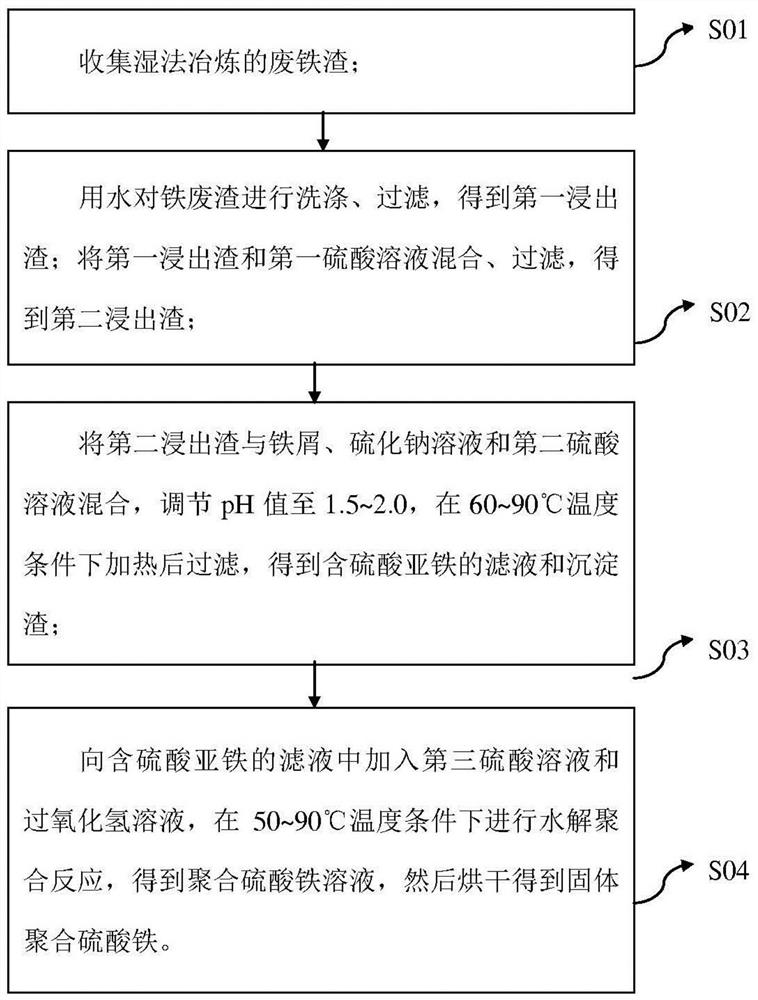
[0041] (1) Prepare raw materials: collect 100g of solid waste iron slag purified in the iron removal workshop, and its composition and content include: Co0.5%, Cu 0.03%, Fe9.4%, Na 0.2%.
[0042] (2) Pretreatment: with a solid-to-liquid ratio of 1g:5ml, the iron slag is washed with pure water for 1h, and then filtered to obtain the first leaching slag. The leaching solution can be recycled and used, and then use 0.5mol / L 95% sulfuric acid The solution is mixed with the first leaching residue after washing, stirred for 1 hour and then filtered to obtain the second leaching residue, and the leaching solution is used to recover cobalt.
[0043] (3) Sulfidation precipitation: add the pretreated second leaching slag into a beaker, add excess scrap iron filings, 5g / L sodium sulfide solution and sulfuric acid solution, keep the pH value of the solution at 2.0, heat to 60 ° C, and react After 3 hours, the solution was filtered to obtain a filtrate containing ferrous sulfate and a prec...
Embodiment 2
[0047] (1) Prepare raw materials: collect 100g of solid waste iron slag purified in the iron removal workshop, and its composition and content include: Co0.8%, Cu0.06%, Fe13.6%, Na 0.3%.
[0048] (2) Pretreatment: with a solid-to-liquid ratio of 1g:5ml, the iron slag is washed with pure water for 1h, and then filtered to obtain the first leaching slag. The leaching solution can be recycled and used, and then use 0.5mol / L 98% sulfuric acid The solution is mixed with the first leaching residue after washing, stirred for 1 hour and then filtered to obtain the second leaching residue, and the leaching residue liquid is used to recover cobalt.
[0049] (3) Sulfidation precipitation: add the pretreated second leaching slag into a beaker, add excess scrap iron filings, 7.5g / L sodium sulfide solution and sulfuric acid solution, keep the pH value of the solution at 1.5, and heat to 70°C, The reaction was carried out for 3 hours, and then the solution was filtered to obtain a filtrate c...
Embodiment 3
[0053] (1) Prepare raw materials: collect 100g of solid waste iron slag purified in the iron removal workshop, and its composition and content include: Co0.8%, Cu0.06%, Fe13.6%, Na0.4%.
[0054] (2) Pretreatment: with a solid-to-liquid ratio of 1g:5ml, the iron slag is washed with pure water for 1h, and then filtered to obtain the first leaching slag. The leaching solution can be recycled and used, and then use 0.5mol / L 98% sulfuric acid The solution is mixed with the first leaching residue after washing, stirred for 1 hour and then filtered to obtain the second leaching residue, and the leaching solution is used to recover cobalt.
[0055] (3) Sulfidation method precipitation: add the pretreated second leaching slag into a beaker, add excess scrap iron filings, 10g / L sodium sulfide solution and sulfuric acid solution, keep the pH value of the solution at 1.5, heat to 90 ° C, and react After 3 hours, the solution was filtered to obtain a filtrate containing ferrous sulfate and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com