FIPV N recombinant protein and obtained colloidal gold test strip for rapidly detecting FIPV infection
A technology of colloidal gold test paper and recombinant protein, applied in biological testing, recombinant DNA technology, DNA / RNA fragments, etc., can solve the problems of high variability and undetectability of S protein, and achieve short cycle, cost saving and low cost detection way effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1FI
[0037]Construction and prokaryotic expression of embodiment 1 FIPV N protein recombinant plasmid
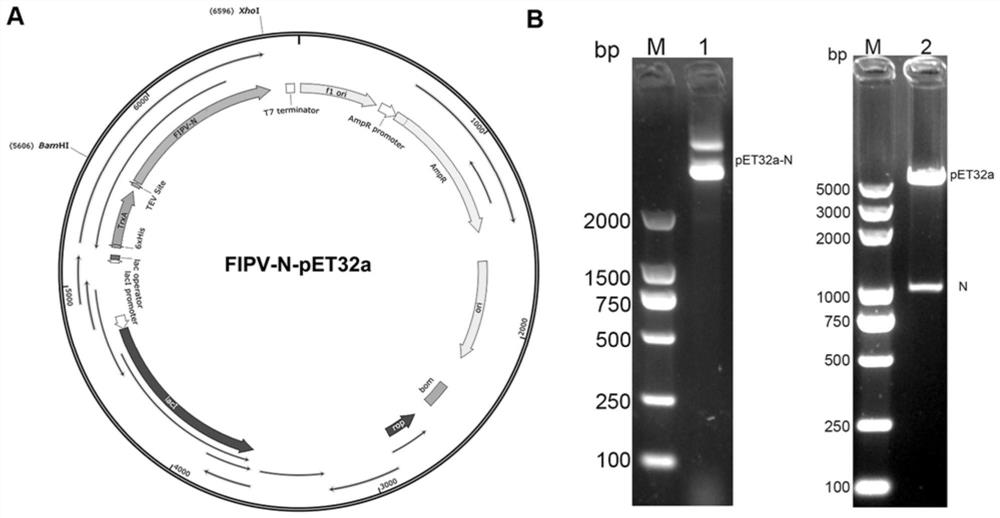
[0038] The protein secondary structure prediction software and protein nuclear localization signal prediction software in Novopro online tool were used to analyze the secondary structure and nuclear localization signal (NLS) of the FIPV N protein sequence of 79-1146 strain (GeneBank ID: DQ 010921.1). Combined with the high antigenic index and α-helix, β-sheet, β-turn enriched regions, the FIPV N expression gene 151-1131bp was selected for codon optimization, and a gene fragment with a size of 1134bp was synthesized. Synthesize his tag, TrxA tag, BamHI and XhoI restriction sites, and add a TEV restriction site after the upstream BamHI site, such as figure 1 As shown in A. The synthetic N gene was digested with BamHI and XhoI and then ligated into the pET32a vector which was digested with the same enzymes. After the pET32a-FIPV N recombinant plasmid expressed in prokaryotic is di...
Embodiment 2F
[0040] Embodiment 2 FIPV N protein purification and identification
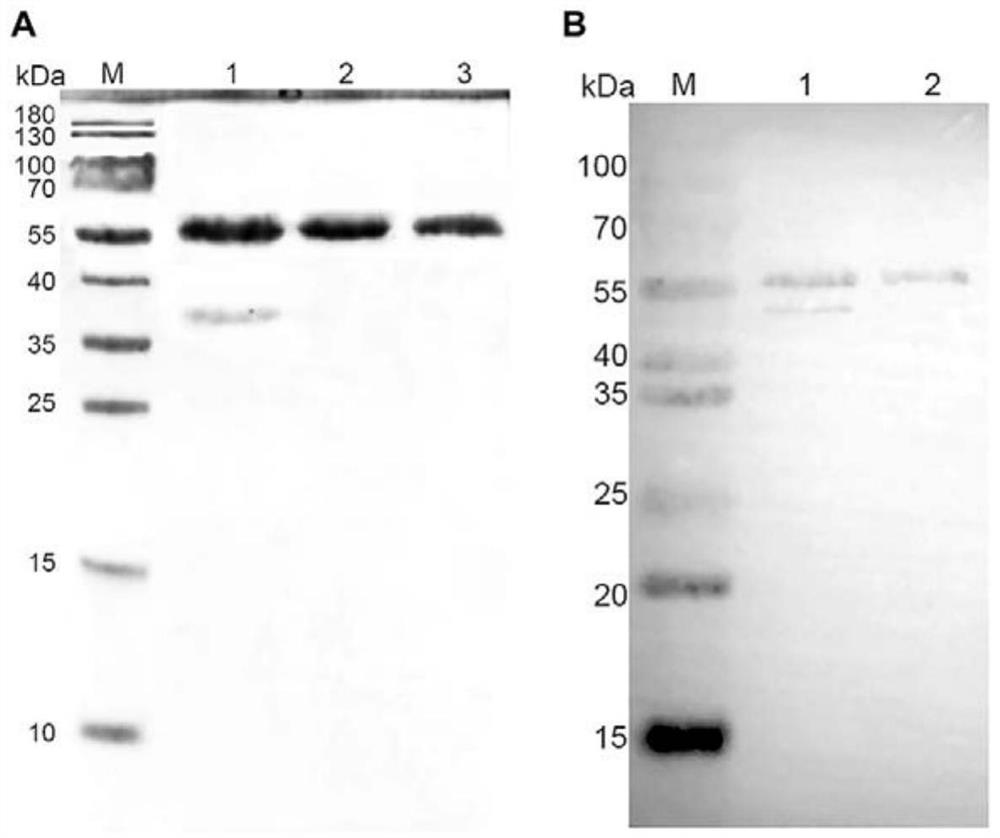
[0041] Add the supernatant protein sample collected in Example 1 to the nickel column equilibrated with 50mM Tris-HCl in advance, incubate for 1h to make the protein fully bind to the nickel column, then let the sample flow out slowly, collect the effluent, and load the sample again . Use 5 times the column volume of 50mM and 100mM imidazole to elute and collect the protein; transfer the collected protein to an ultrafiltration tube, 4°C, 3000r / min ultrafiltration concentration, take 8μl of the concentrated protein, add 2μL 5 ×The protein loading buffer was mixed and denatured for 10 minutes, and 8 μl samples were taken for SDS-PAGE identification.
[0042] SDS-PAGE electrophoresis showed that in the inclusion body, 50 and 100mM imidazole elution concentrate, the target band appeared at about 55kDa ( figure 2 A). Moreover, a relatively pure FIPV N protein was obtained after 100 mM elution of the concentrat...
Embodiment 3F
[0043] Example 3 FIPV N protein quantification
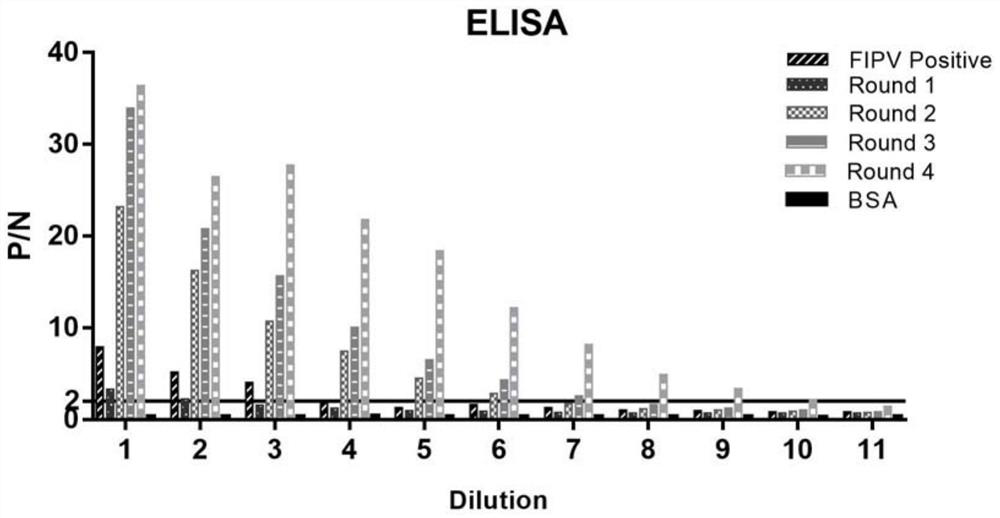
[0044] Take 25 μL of 8 serially diluted BSA protein standard samples, respectively named A, B, C, D, E, F, G, H, and take 25 μL of FIPV N protein samples to be tested, and add them to the microwell plate in turn. Add 200 μL of working solution to each well, and shake on the shaker at room temperature for 30 seconds to make it fully mixed. Seal the microwell plate with a ziplock bag and place it at 37°C for 30 minutes. Cool the 96-well plate to room temperature, read the absorbance value of the sample at 562 nm with a microplate reader, and calculate the concentration of the protein to be tested according to the obtained linear equation. The concentrations of N protein and its two serial dilutions were 27.27, 13.56, 5.65, 3.83, 2.46, 0.63 and 0.31 μg / mL, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com