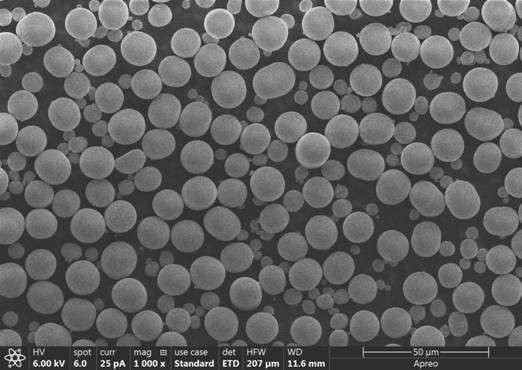
Nickel-cobalt-boron precursor material, preparation method and nickel-cobalt-boron positive electrode material
A cathode material and precursor technology, applied in the field of lithium-ion battery materials, can solve the problems of material capacity attenuation, impedance increase, penetration, etc., and achieve the effects of reducing impedance, suppressing polarization, and improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment includes the following steps:
[0047] (1) Preparation of Ni 0.9 co 0.08 B 0.02 (OH) 2 Precursor
[0048] ① Solution preparation: 118.28 kg of nickel sulfate hexahydrate, 11.25 kg of cobalt sulfate heptahydrate, 0.70 kg of boron oxide and hot pure water were fully mixed and dissolved to prepare 200 L of solution A, the concentration of which was 2.5 mol / L, and the concentration of nickel, cobalt and boron Molar ratio Ni:Co:B=9.0:0.8:0.2; take 25% industrial ammonia water and configure it as 7.5 mol / L solution B, the volume is 100 L, and the molar ratio to solution A is 1.5; 32% industrial hydrogen Sodium oxide was mixed with distilled water to prepare 12.5 mol / L solution C with a volume of 100 L and a molar ratio of 2.5 to solution A; solutions A, B and C were kept at a constant temperature of 40 °C.
[0049] ②Preparation of reaction kettle bottom liquid D: In a 300 L reaction kettle, first add hot pure water to 1 / 2 of the volume of the reaction ket...
Embodiment 2
[0060] This embodiment includes the following steps:
[0061] (1) Preparation of Ni 0.75 co 0.2 B 0.05 (OH) 2 Precursor:
[0062] ① Solution preparation: 157.71 kg of nickel sulfate hexahydrate, 44.98 kg of cobalt sulfate heptahydrate, 2.78 kg of boron oxide and hot pure water were fully mixed and dissolved to prepare 400 L of solution A with a concentration of 2.0 mol / L. Molar ratio Ni:Co:B=7.5:2.0:0.5; take 25% industrial ammonia water and prepare 8.0 mol / L solution B, the volume is 100 L, and the molar ratio of solution A is 1; 32% industrial hydrogen Mix sodium oxide and distilled water to prepare 9.6 mol / L solution C with a volume of 100 L and a molar ratio of 1.2 to solution A; keep solutions A, B, and C at a constant temperature of 45°C.
[0063] ②Preparation of reaction kettle bottom liquid D: In a 500 L reaction kettle, first add hot pure water to 1 / 2 of the volume of the reaction kettle, control the temperature in the kettle to 55°C, stir at 350 rpm, and then co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface energy | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Surface energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com