Panax japonicus saponin glucoside hydrolase and application thereof in production of notoginsenoside R1
A technology of glycoside hydrolase and ginsenoside, applied in the production of ginger-like notoginseng saponin R1, the field of glycoside hydrolase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
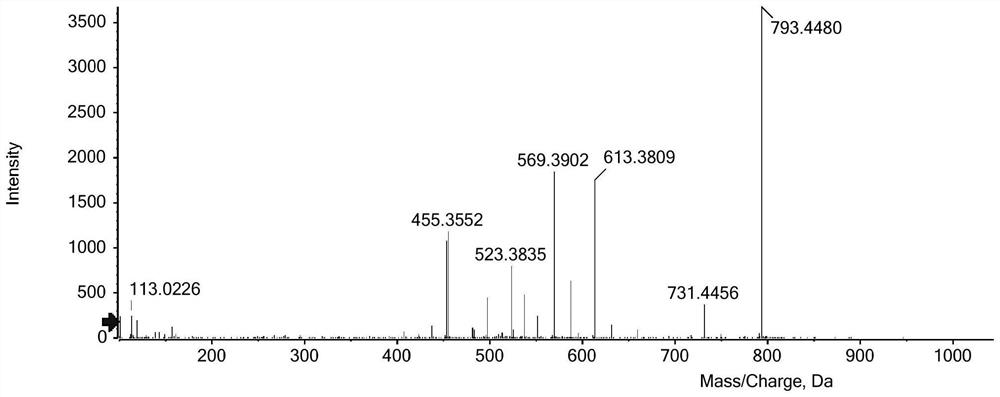
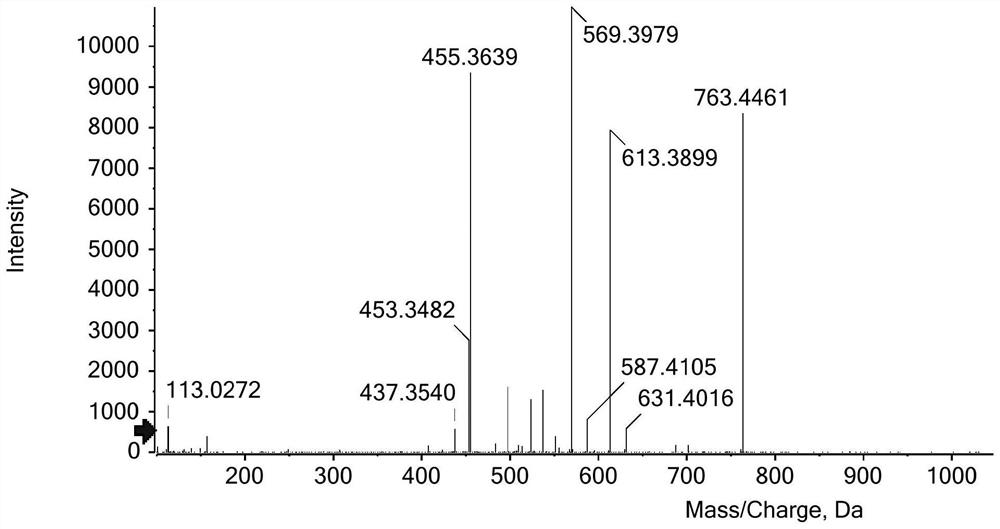
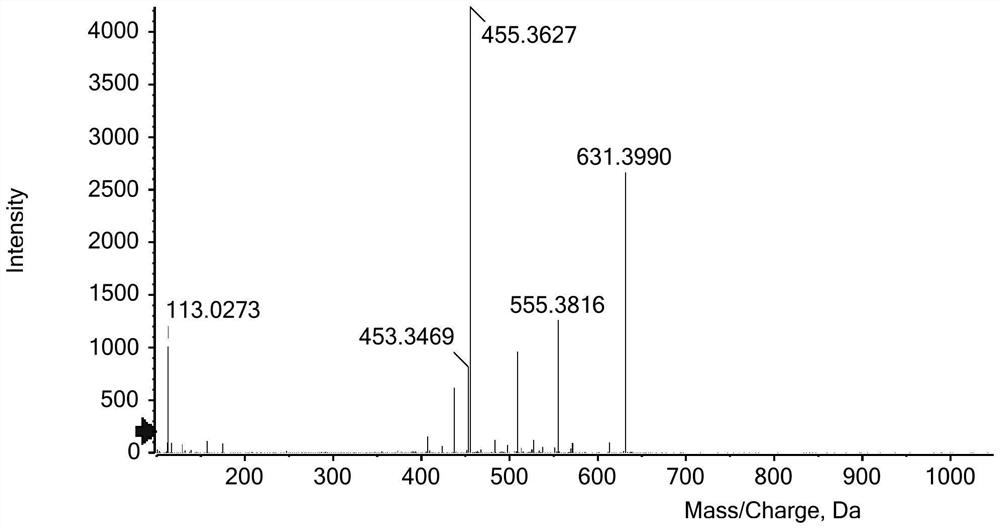
Image
Examples
Embodiment 1
[0055] Embodiment 1, cloning of bamboo ginseng saponin glycoside hydrolase and its coding gene
[0056] Genomic DNA extracted from Paenibacillus lactis was used as a template, and 5′-CGCGGATCCATGAGAAACCATACTTTAGATACG-3′(Forward) and 5′-CCCAAGCTT TCAGCTTCTACGGTATCTCTTC-3′(Reverse) were used as primers for polymerase chain reaction PCR amplification.
[0057] PCR system: 12.5 μL of 2×Taq Mixture, 0.5 μL of upstream and downstream primers (10 μm), 0.5 μL of genome and ddH 2 O 11.5 μL.
[0058] The PCR conditions were as follows: pre-denaturation at 95°C for 3 minutes, followed by 30 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 2 min; finally, extension at 72°C for 10 min.
[0059] The above PCR products were analyzed by agarose gel electrophoresis, the gel was tapped and the target band was recovered with a kit.
[0060] The recovered products were digested with the pET-28a(+) vector with restriction endonucleases BamHI and HindIII respectively (37°C, 6h); the digested ...
Embodiment 2
[0062] Embodiment 2, expression and purification of recombinant PlGH03
[0063] Inoculate the single colony of BL21(DE3) / pET-28a(+)-Pl3 obtained above into LB liquid medium containing kanamycin (final concentration: 50 μg / mL), culture at 37°C for 12 hours, and take 1 mL of bacteria solution was added to 100 mL of fresh LB liquid medium (containing kanamycin at a final concentration of 50 μg / mL), and cultured at 37°C until OD 600 When it reached 0.4, IPTG (final concentration: 0.2mM) was added to the culture medium, and induction culture was continued at 16°C for 24h. Collect the fermentation broth, and centrifuge at 8000rpm for 5min to collect the bacteria. Wash twice with 50 mL of normal saline, and collect the cells by centrifugation.
[0064] Resuspend the bacteria with 10mL solution A (10mM pH7.4 sodium phosphate buffer, containing 20mM imidazole, 500mM NaCl). In an ice-water bath, crush the bacterial cells with an ultrasonic crusher (400W, work for 4s and pause for 6s,...
Embodiment 3
[0068] The optimal temperature of embodiment 3, recombinase PlGH03
[0069] Take a certain amount of recombinant enzyme PlGH03 and p-nitrophenyl-β-D-glucoside with a final concentration of 2 mM, add pH 8.050 mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer to a volume of 0.5 mL, and place in 25- After reacting in a water bath at 55°C for 5 min, add 0.5 mL of 1 mM Na 2 CO 3 The solution terminated the reaction, and the absorbance value at 405 nm was measured, and the highest activity was taken as 100%. The relative activity results are shown in Table 2, and the enzyme has the highest reactivity at 50°C.
[0070] The optimal temperature of table 2 recombinase PlGH03
[0071]
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com