Viscous biological sample liquefaction release combination product, kit, liquefaction release method and nucleic acid extraction, amplification and detection method
A technology of biological samples and combined products, which is applied in the direction of biochemical equipment and methods, microbial measurement/inspection, etc., can solve the problems of complex operation, insufficient, sample mixing and liquefaction, etc., and achieve good release effect and good compatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
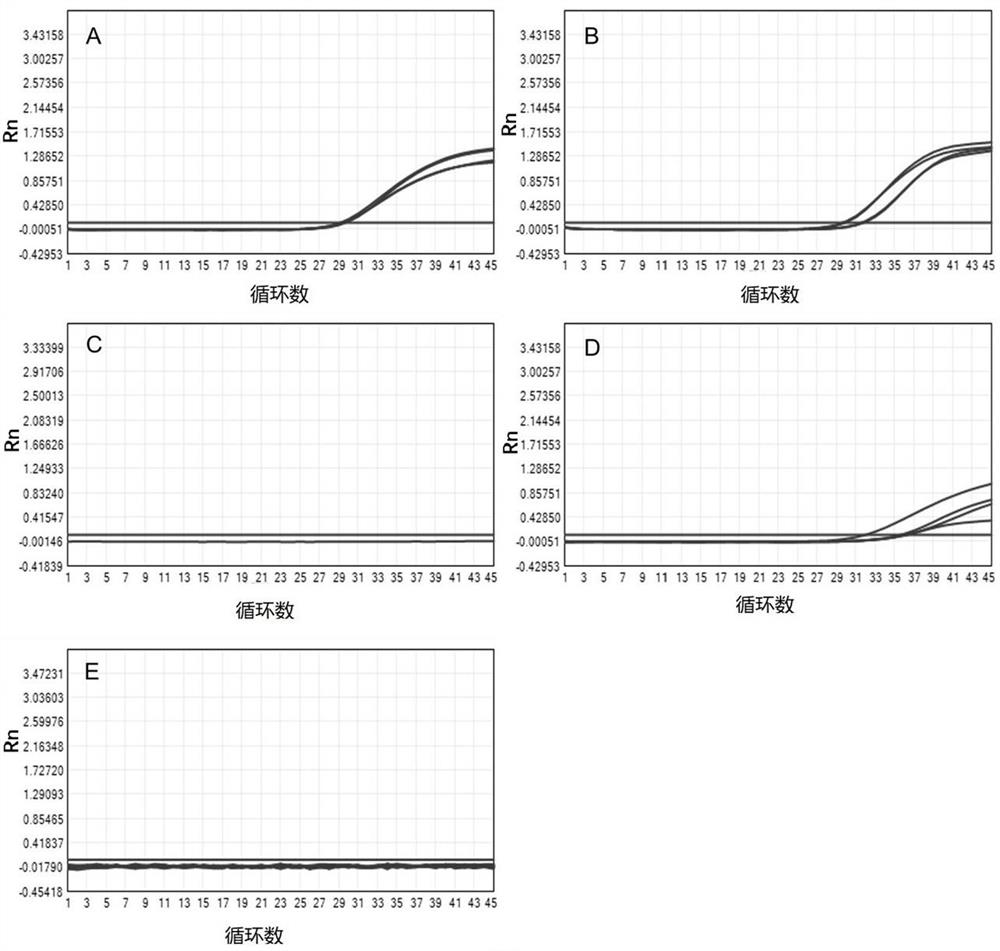
[0262] Embodiment 1. Liquefaction effect test
[0263] In this embodiment, the liquefaction reagent of the experimental group was prepared by using guaifenesin, sodium hydroxide and zirconia beads. The final concentration was 100mM guaifenesin, 10mM sodium hydroxide, and 1g / mL zirconia beads (diameter: 1mm). The solvent used to prepare the reagent was purified water, which was recorded as the experimental group.
[0264] Collected 4 cases of visually viscous sputum samples (I, II, III, IV), each of these sputum samples was fully mixed separately, and 6 2mL samples were taken from each case, numbered 1~6. spare.
[0265] Add 6 mL of experimental group reagent to sample No. 1;
[0266] Add 6 mL of dithiothreitol solution ((2% (w / v) DTT) to sample No. 2;
[0267] Add 6 mL of sodium hydroxide solution (0.1M) to sample No. 3;
[0268] Add 6mL of reagents prepared with a final concentration of 100mM acetylcysteine, 10mM sodium hydroxide, and 1g / mL zirconia beads (diameter: 1mm) ...
Embodiment 2
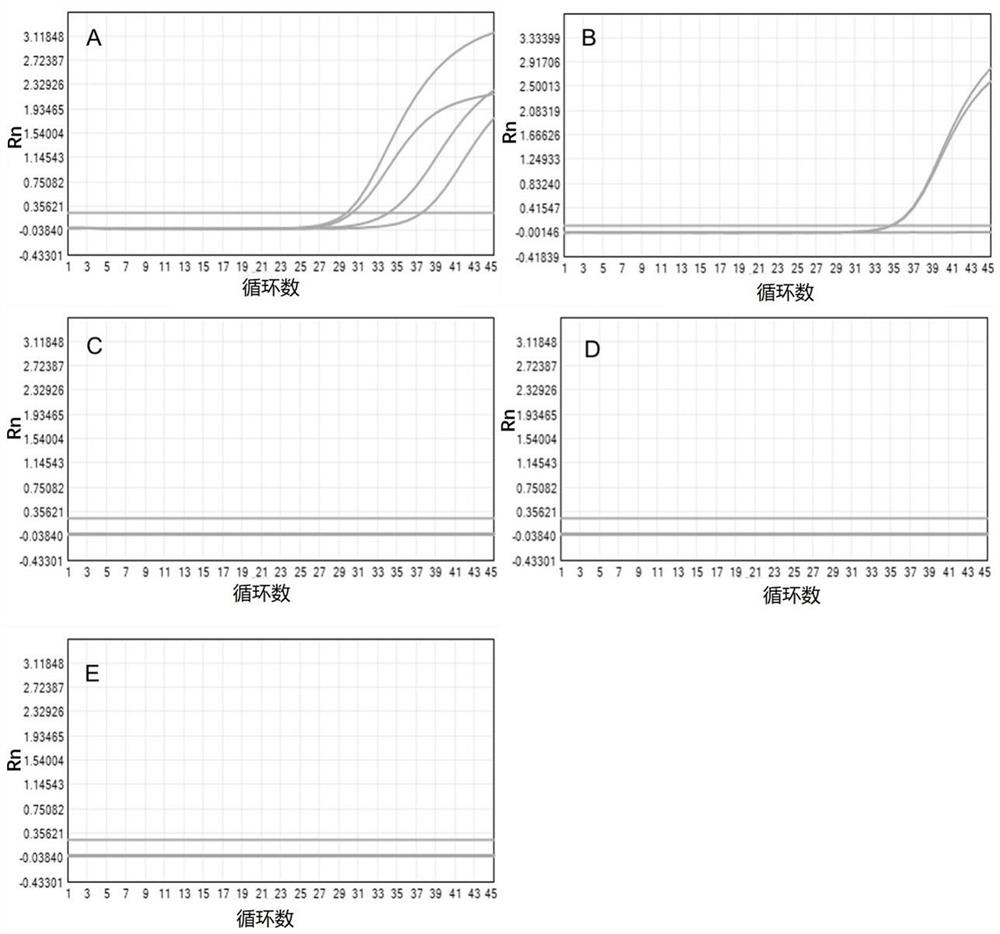
[0278] Example 2. Effect test of different concentrations of liquefied components combined with zirconia beads
[0279] In this embodiment, the liquefaction reagent of the experimental group consists of one or more of the following components: guaifenesin, sodium hydroxide, and zirconia beads.
[0280] Experimental group 1: The final concentration was 100mM guaifenesin, 10mM sodium hydroxide, 1g / mL zirconia beads (diameter 1mm), and the solvent used to prepare the reagent was purified water.
[0281] Experimental group 2: The final concentration was 1mM guaifenesin, 1000mM sodium hydroxide, 1g / mL zirconia beads (diameter 1mm), and the solvent used to prepare the reagent was purified water.
[0282] Experimental group 3: The final concentration was 1mM guaifenesin, 1g / mL zirconia beads (diameter: 1mm), and 0.1mM sodium hydroxide. The solvent used for preparing the reagent was purified water.
[0283] The collected 4 cases of viscous sputum samples (I, II, III, IV) were collect...
Embodiment 3
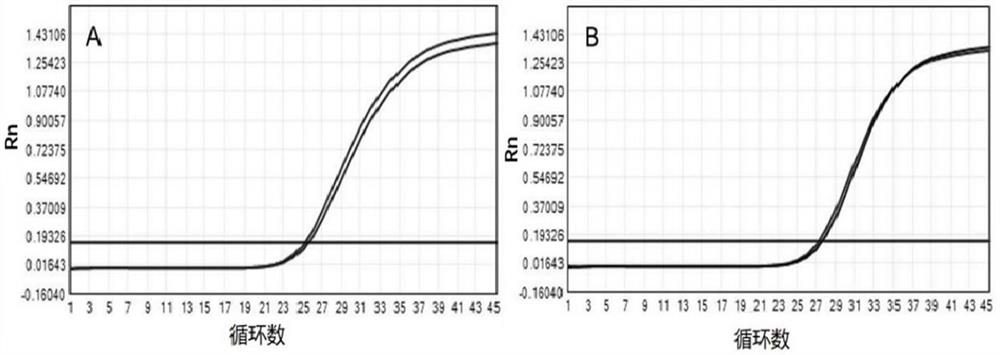
[0292] Example 3. Comparison with other methods for rapid detection of sputum samples after liquefaction
[0293] In this embodiment, the liquefaction reagent of the experimental group was prepared by using guaifenesin, sodium hydroxide and zirconia beads. The final concentration was 100mM guaifenesin, 10mM sodium hydroxide, and 1g / mL zirconia beads (1mm in diameter). The solvent used to prepare the reagent was purified water, which was used as the experimental group.
[0294] Collect 4 cases of visually viscous sputum samples (I, II, III, IV), mix each of these sputum samples fully separately, and take 5 2mL samples from each case, numbered 1~5. spare.
[0295] Add 6 mL of experimental group reagent to No. 1 sample; add 2.5 g of guanidine hydrochloride + 0.5 g of acetylcysteine + 2 g of polypropylene particles to No. 2 sample (the method disclosed in Patent Document No. CN108949748A); Add 6mL of dithiothreitol solution (2%DTT); add 6mL trypsin solution (buffer system cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The way to | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com