Organic solar cell acceptor material, preparation method thereof and organic solar cell
A solar cell and acceptor material technology, applied in the field of organic solar cells, can solve the problems of narrow absorption range, low mobility, and wide optical bandgap, and achieve the effects of wide absorption range, improved mobility, and narrow optical bandgap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
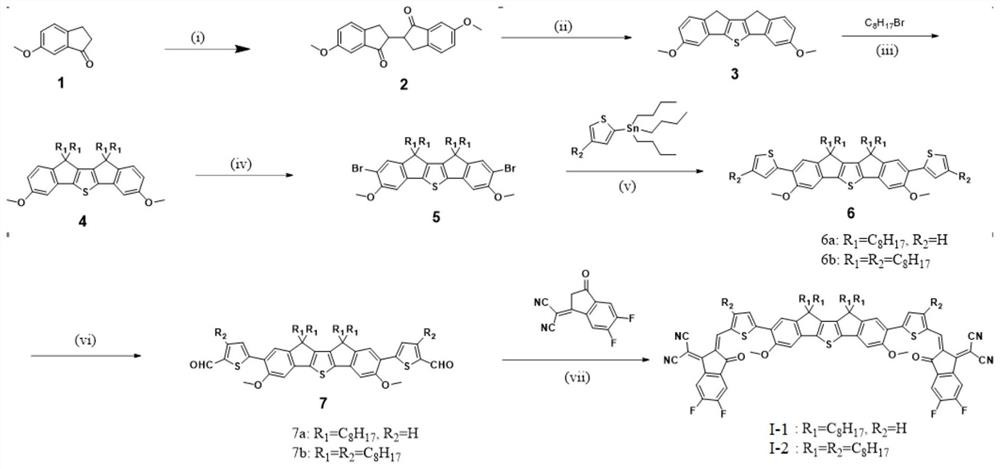
[0067] In a second aspect, the present application also provides a method for preparing an organic solar cell acceptor material, such as figure 1 shown, including the following steps:
[0068] (1) Synthesis of compound 2: under the protection of an inert atmosphere, dropwise add the tetrahydrofuran solution containing compound 1 to the tetrahydrofuran solution containing NaH. Compound 1 is 6-methoxy-1-indanone. After the reaction, cool to room temperature , the reaction solution was distilled under reduced pressure, extracted, dried, filtered, and then purified by silica gel column chromatography to obtain compound 2.
[0069] As an optional technical solution of this application, during the synthesis of compound 2, the reaction temperature is controlled at -78°C, and an appropriate amount of CuCl can also be added 2 , THF solution can also be other more polar solvents, as long as it is a solvent with good solubility to the raw materials, which is beneficial to the reaction a...
Embodiment 1
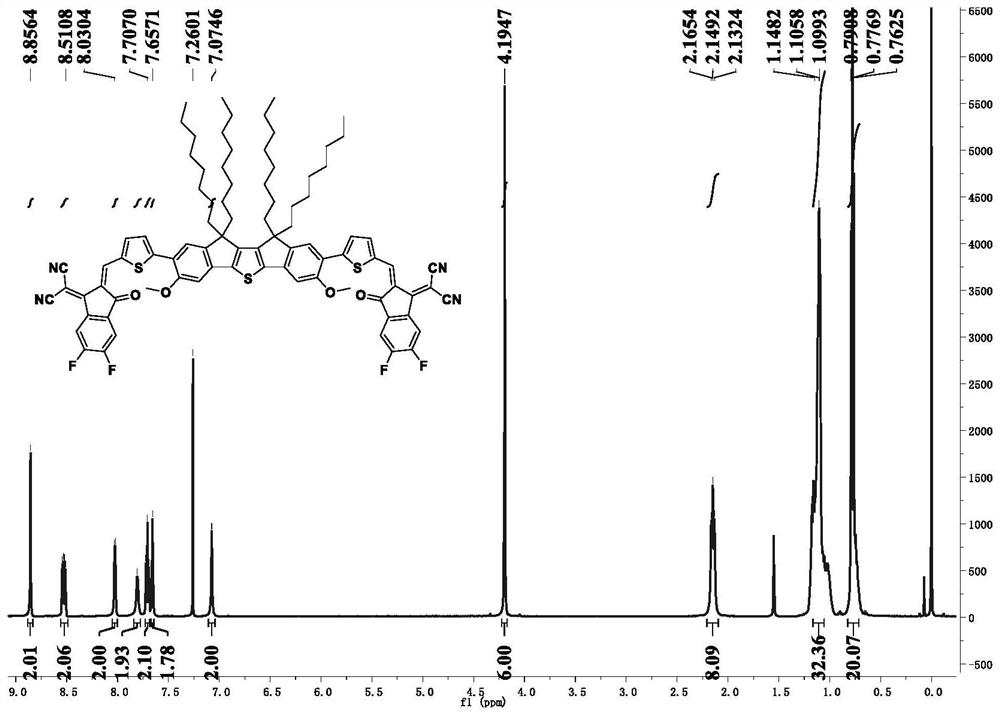
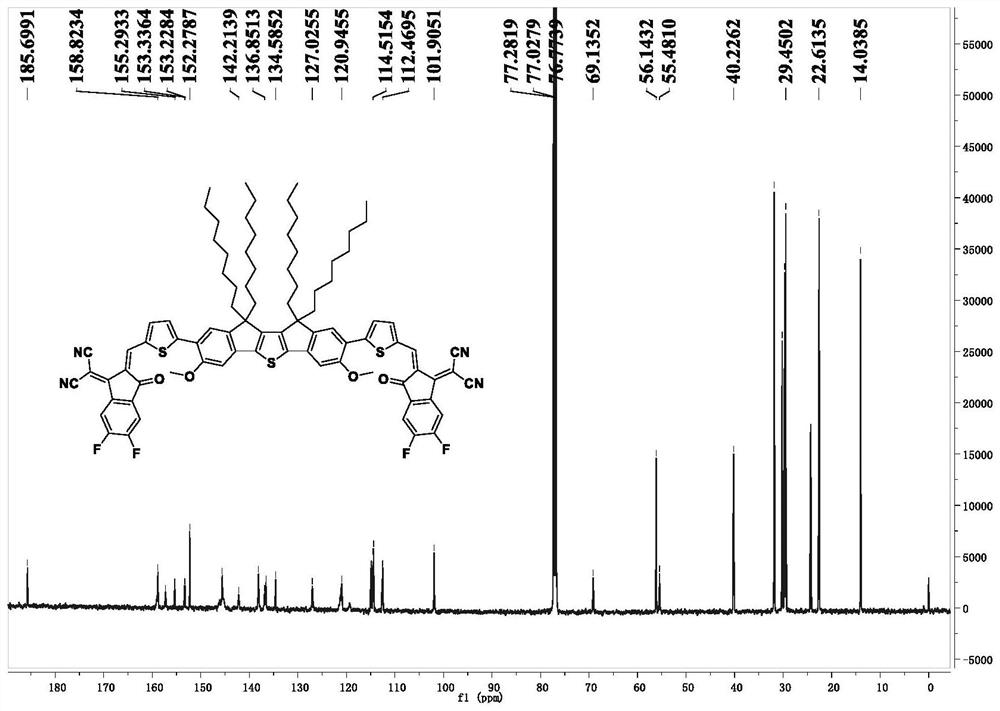
[0108] The preparation method of the compound shown in the formula (I-1) of the present embodiment comprises the following steps:
[0109] (1) Synthesis of Compound 2:
[0110] 6-Methoxy-1-indanone was selected as compound 1, which was purchased from Sarn Chemical Technology (Shanghai) Co., Ltd.
[0111] Add compound 1 (5 g, 0.031 mol) dissolved in ultra-dry reagent tetrahydrofuran solution (100 mL) into a 125 mL constant-pressure dropping funnel, and add NaH (1.85 g, 1.85 g, containing 60% silicone oil), at room temperature, degassing several times to fill the system with nitrogen, and then under the atmosphere of nitrogen protection, the solution containing compound 1 was added dropwise to the tetrahydrofuran solution containing NaH, and stirred at room temperature until no longer generate H 2 until. After the reaction was completed, deionized water was slowly added to quench, the tetrahydrofuran solvent was first spin-dried, and then extracted three times with dichlorome...
Embodiment 2
[0133] The preparation method of the compound shown in the formula (I-2) of the present embodiment comprises the following steps:
[0134] (1) Synthesis of Compound 2:
[0135] 6-Methoxy-1-indanone was selected as compound 1, which was purchased from Sarn Chemical Technology (Shanghai) Co., Ltd.
[0136] Add compound 1 (5 g, 0.031 mol) dissolved in ultra-dry reagent tetrahydrofuran solution (100 mL) into a 125 mL constant-pressure dropping funnel, and add NaH (1.85 g, 1.85 g, containing 60% silicone oil), at room temperature, degassing several times to fill the system with nitrogen, and then under the atmosphere of nitrogen protection, the solution containing compound 1 was added dropwise to the tetrahydrofuran solution containing NaH, and stirred at room temperature until no longer generate H 2 until. After the reaction was completed, deionized water was slowly added to quench, the tetrahydrofuran solvent was first spin-dried, and then extracted three times with dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com