Preparation method of SnO2/Fe3O4 composite nano catalyst
A nano-catalyst, hydrated iron oxide technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve the problem of large difference in crystal structure and achieve good magnetic properties High performance, easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: SnO 2 / Fe 3 o 4 Composite nanocatalyst preparation
[0025] Add 0.5g of α-FeOOH powder to 50mL of SnCl with a concentration of 0.2mol / L 2 In aqueous solution (hydrated iron oxide (FeOOH) powder and stannous chloride (SnCl 2 ) The weight ratio between is 1:3.79), after ultrasonic dispersion for 5min, add 0.2g NaOH solid, and after it is completely dissolved, the precursor FeOOH-Sn 2+ -NaOH-H 2 O, the pH value of the system is 8.
[0026] The precursor FeOOH-Sn 2+ -NaOH-H 2 O was transferred into the reaction kettle, and the hydrothermal reaction was carried out in an oven at 180°C for 24 hours. After the reaction, the product was taken out for washing and drying.
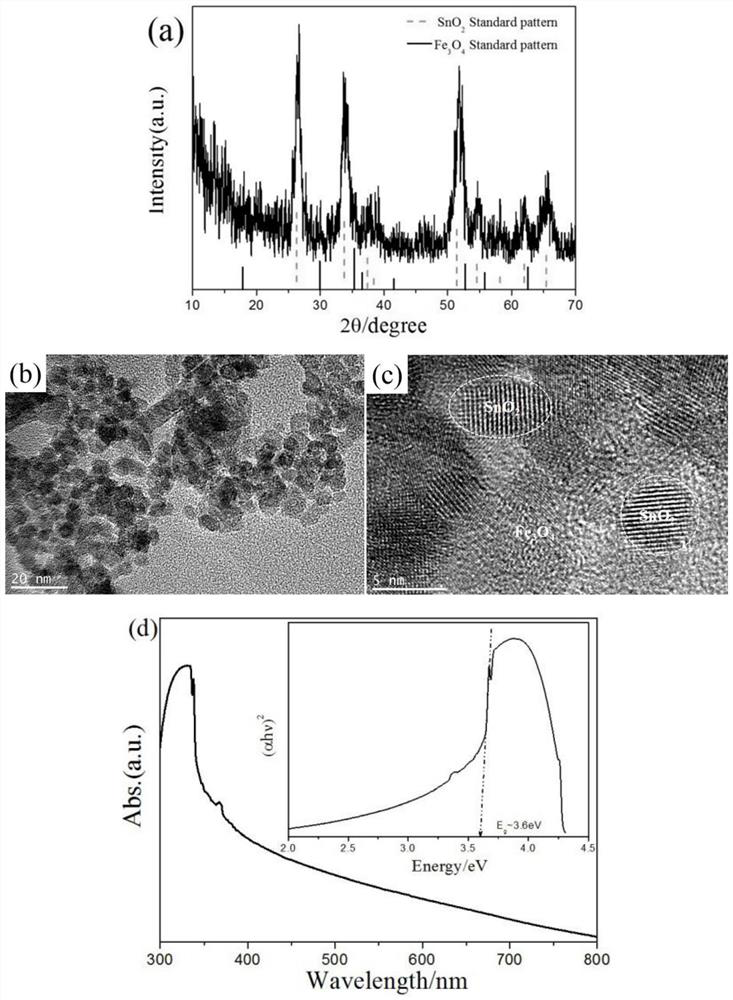
[0027] First, the phase analysis of the product was carried out by means of X-ray diffraction and compared with the bulk SnO 2 Standard XRD diffraction (PDF, No.040783) and Fe 3 o 4 After comparison with the standard XRD diffraction (PDF, No.040783), it is known that the product obtained ...
Embodiment 2
[0030] Embodiment 2: the impact of FeOOH dosage on product form, composition and performance
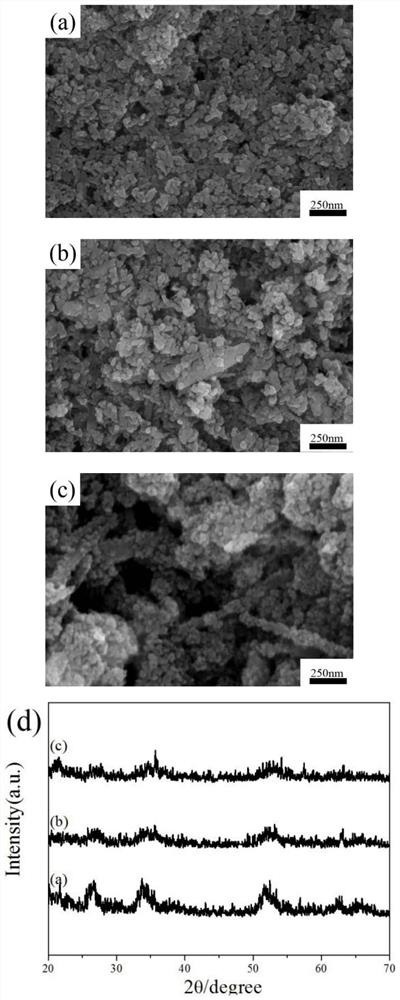
[0031] The preparation method is the same as in Example 1, the addition amount of α-FeOOH powder is adjusted to 0.1g, 0.3g, and 0.7g respectively, and the morphology of the product is observed by SEM. It can be seen that with the gradual increase in the amount of FeOOH powder used in the reaction system increasing, the morphology of the product gradually changed from random nanoparticles to rod-like particles ( figure 2 a~c).
[0032]The phase analysis of the product by means of X-ray diffraction shows that too little or too much FeOOH powder will make the final composite material phase impure. It can be seen that when other experimental conditions are the same, the amount of FeOOH powder used has a great influence on the morphology and structure of the final product ( figure 2 d). When the amount of α-FeOOH powder added is between 0.3-0.5 g, the prepared product has higher puri...
Embodiment 3
[0033] Embodiment 3: the impact of NaOH dosage on product morphology and magnetic properties
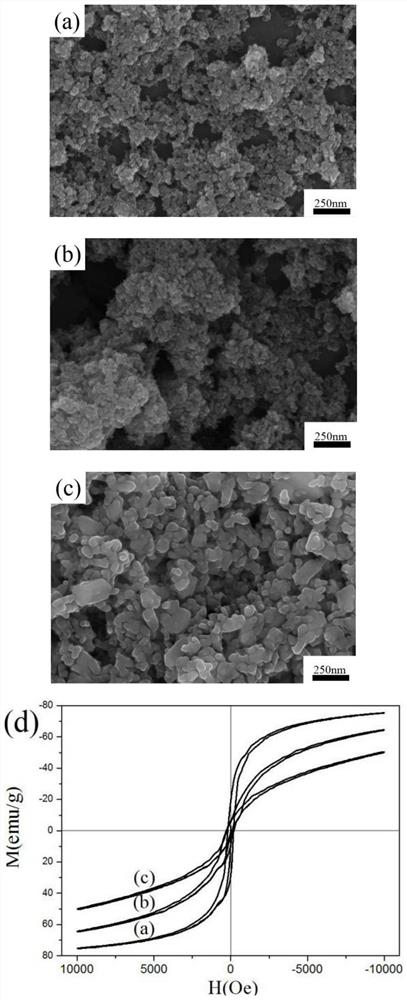
[0034] The preparation method is the same as in Example 1, and the addition amount of NaOH is adjusted to be 0.1g, 0.4g, and 0.6g respectively.
[0035] In the reaction system, the amount of NaOH used has a great influence on the formation of the phase and the particle size of the product, thus affecting the performance of the final product. from image 3 As a result, it can be seen that as the amount of NaOH used increases gradually, the particle size of the product increases gradually ( image 3 a~c), the macroscopic magnetism of the product is also gradually enhanced ( image 3 d).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com