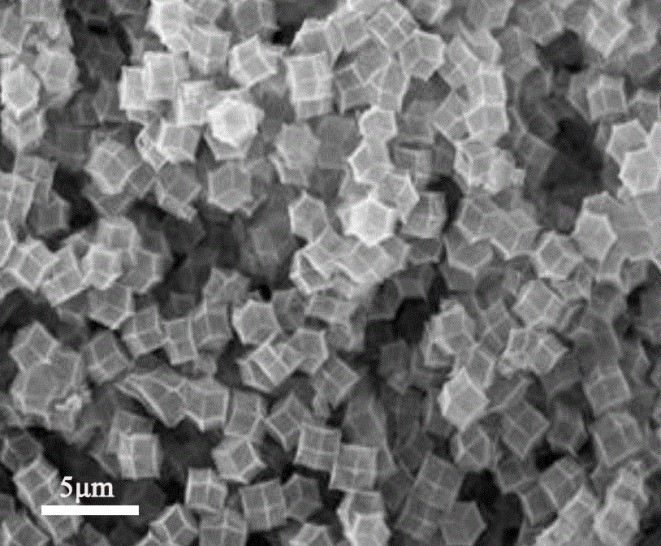
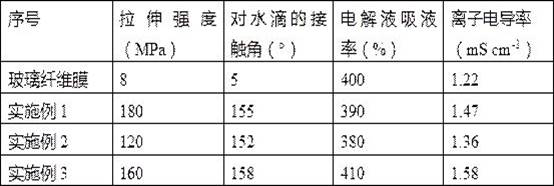
Preparation method of super-hydrophobic super-oleophylic lithium air battery composite diaphragm
A lithium-air battery and composite diaphragm technology, applied in battery pack components, chemical instruments and methods, membranes, etc., can solve problems such as corrosion of lithium metal negative electrodes, safety problems, etc., to prevent evaporation, inhibit the formation of lithium dendrites, The effect of suppressing the shuttle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Add 2.0 g of methyl 2-hydroxy-4-iodobenzoate to 3 mL of acetic anhydride, then add 85 μL of concentrated sulfuric acid to obtain a mixed solution, and magnetically stir the mixed solution at 80° C. for 12 h; after cooling to room temperature , add 15mL deionized water to the mixed solution, and then use 20mL CHCl 3 Extracted 3 times; the obtained 3 extracts were mixed, and then passed through a short silica gel column to remove part of the inorganic salt impurities, and the obtained liquid was sequentially washed with saturated NaHCO 3 solution washing, with MgSO 4 Product A was obtained as a white solid after drying, filtration and removal of solvent under vacuum;
[0027] in N 2 Under atmosphere, mix 1.60g product A, 3.00g active copper, 4mL N,N-dimethylformamide, then heat the mixed solution to 155°C for 10h; filter the reacted mixed solution while hot, and then collect The filtrate was poured into 40 mL of deionized water and stirred vigorously, the fo...
Embodiment 2
[0033] Example 2: Add 2.0 g of methyl 3-hydroxy-4-iodobenzoate to 2.8 mL of butenedioic anhydride, then add 80 μL of concentrated sulfuric acid to obtain a mixed solution, and magnetically stir the mixed solution at 80° C. for 12 h; leave to cool After reaching room temperature, 12 mL of deionized water was added to the mixed solution, followed by 20 mL of CH 2 ClCH 2 Cl extraction 3 times; the 3 extracts obtained were mixed, and then passed through a short silica gel column to remove part of the inorganic salt impurities, and the obtained liquid was successively passed through saturated NaHCO 3 solution washing, with Na 2 SO 4 Product A was obtained as a white solid after drying, filtration and removal of solvent under vacuum;
[0034] in N 2 Under the atmosphere, mix 1.70g of product A, 3.00g of active nickel, and 4mL of N,N-dimethylformamide, and then heat the mixed solution to 160°C for 12h; filter the reacted mixed solution while it is hot, and then collect The filtr...
Embodiment 3
[0040] Example 3: Add 2.2 g of methyl 4-hydroxy-3-iodobenzoate to 2.8 mL of phthalic anhydride, then add 80 μL of concentrated sulfuric acid to obtain a mixed solution, and magnetically stir the mixed solution at 85° C. for 15 h; After cooling to room temperature, 13 mL of deionized water was added to the mixed solution, and then 20 mL of C 2 HCl 5 Extracted 3 times; the obtained 3 extracts were mixed, and then passed through a short silica gel column to remove part of the inorganic salt impurities, and the obtained liquid was sequentially washed with saturated NaHCO 3 solution washing, with Na 2 SO 4 and CaSO 4 Product A was obtained as a white solid after drying, filtration and removal of solvent under vacuum;
[0041] in N 2 Under the atmosphere, mix 1.80g of product A, 3.00g of active titanium, and 4mL of N,N-dimethylacetamide, and then heat the mixed solution to 150°C for 10h; filter the reacted mixed solution while it is hot, and then collect The filtrate was poured ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com