Aryl-oxazole-oxazoline compound as well as preparation method and application thereof
An oxazoline and compound technology, applied in the field of aryl-oxazole-oxazoline compounds and their preparation, can solve problems such as endangering human health and food safety, and achieve excellent inhibitory activity, novel structure and antibacterial activity improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The present invention also provides the preparation method of aryl-oxazole-oxazoline compound described in above-mentioned technical scheme, comprises the following steps:
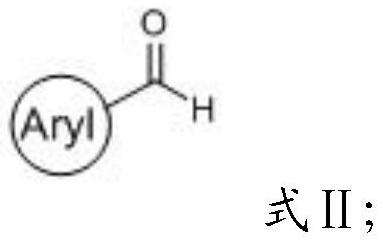
[0047] (1) mixing the compound having the structure shown in formula II, triethylamine, L-serine methyl ester hydrochloride, tetrahydrofuran and molecular sieves for condensation reaction to obtain the first reaction precursor;
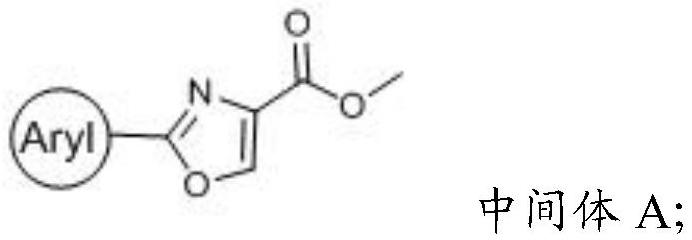
[0048] (2) The first reaction precursor obtained in the step (1), trichlorobromomethane, 1,8-diazabicycloundec-7-ene and dichloromethane are mixed for condensation reaction to obtain an intermediate A;
[0049]
[0050] (3) The intermediate A obtained in the step (2), sodium hydroxide, methanol and water are mixed for a hydrolysis reaction to obtain a second reaction precursor;
[0051] (4) The second reaction precursor obtained in the step (3), 4-dimethylaminopyridine, aminoalcohol, methylene chloride and 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride ...
Embodiment 1
[0097]
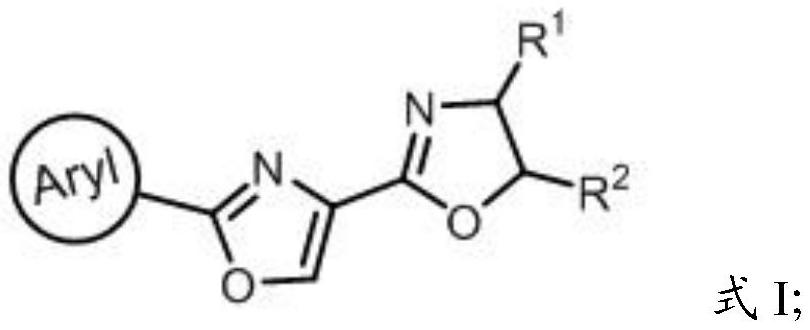
[0098] Aryl-oxazole-oxazoline compound (compound C1, has in the chemical structure shown in formula I is phenyl, R 1 is S-phenyl, R 2 is a hydrogen atom) preparation method
[0099] (1) first L-serine methyl ester hydrochloride (5.9g, 37.7mmol) and tetrahydrofuran (60mL) are mixed to obtain the tetrahydrofuran (60mL) solution of L-serine methyl ester hydrochloride (5.9g, 37.7mmol), then Triethylamine (10.5mL, 75.4mmol) and benzaldehyde (4.0g, 37.7mmol) were successively added to a solution of L-serine methyl ester hydrochloride (5.9g, 37.7mmol) in tetrahydrofuran (60mL) at room temperature, And add 3A molecular sieves (0.5g) and stir at room temperature to carry out condensation reaction, TLC tracking monitoring, after 12h, the reaction is complete, the product of the condensation reaction is successively filtered and concentrated to obtain 7.0g light yellow first reaction precursor;
[0100] (2) Mix the first reaction precursor (7.0g, 33.8mmol) obtained in the...
Embodiment 2~24
[0105] Compounds C2-C24 were prepared according to the method of Example 1;
[0106] The difference from Example 1 is that the raw materials benzaldehyde and L-serine methyl ester hydrochloride were replaced with raw materials corresponding to compounds C2-C24.
[0107] Table 1 The structures of the compounds prepared in Examples 1 to 24 and the statistics of the H NMR and C NMR spectra detected
[0108]
[0109]
[0110]
[0111]
[0112]
[0113]
[0114]
[0115]
[0116]
[0117]
[0118]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com