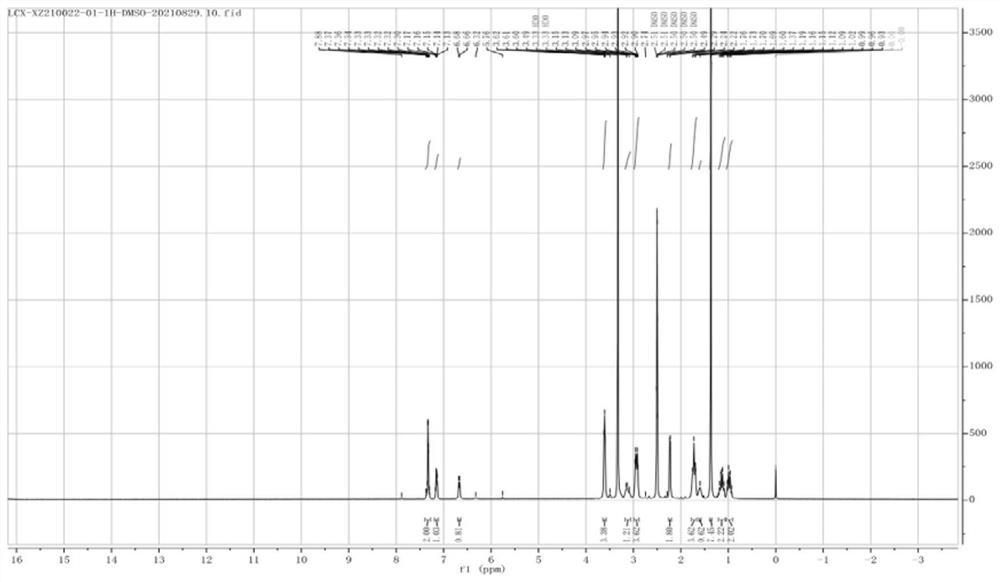
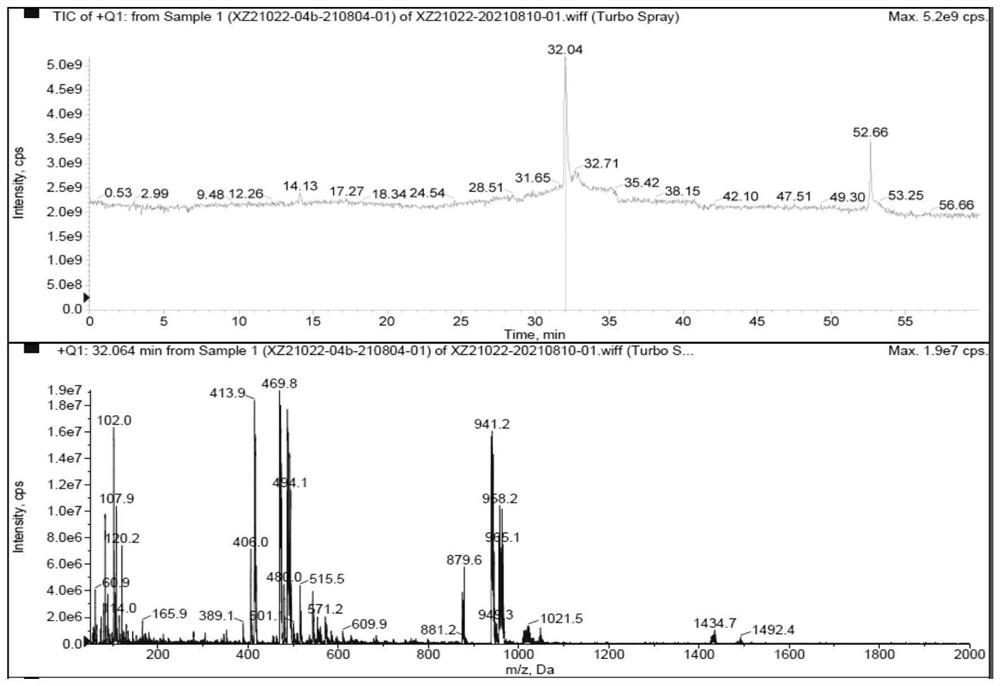
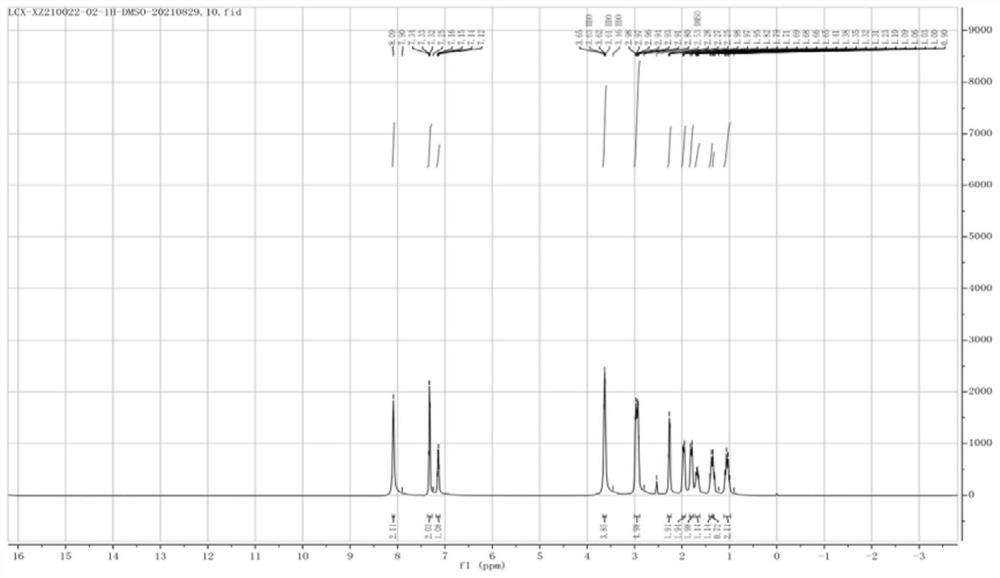
Preparation method of cariprazine and intermediate compound
A cariprazine and compound technology, which is applied to the preparation method of cariprazine and the field of intermediate compounds, can solve the problems of long reaction time, long reaction steps and high temperature, and achieves shortening of process steps, improvement of total yield, The effect of ensuring purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The first aspect of the present invention provides a kind of preparation method of cariprazine, it comprises:
[0047] (1) React with trans-(N-Boc-4-aminocyclohexyl)acetic acid and 1-(2,3-dichlorophenyl)piperazine as raw materials to obtain trans-N-tert-butoxycarbonyl-4 -{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexylamine (compound shown in formula 1);
[0048](2) Make the resulting trans N-tert-butoxycarbonyl-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}- Cyclohexylamine was deprotected by acid to give trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclo Hexylamine (compound shown in II);
[0049] (3) The resulting trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexylamine The reduction reaction obtains the trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl}-cyclohexylamine (compound shown in III) ;
[0050] (4) Make the resulting trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-...
Embodiment approach
[0052] According to some embodiments of the present invention, the activation is to react trans-(N-Boc-4-aminocyclohexyl)acetic acid with the activator to generate acid halide, acid anhydride or carbonylimidazole intermediate compound.
[0053] According to some preferred embodiments of the present invention, the activator is thionyl chloride, oxalyl chloride, isobutyl chloroformate or carbonyldiimidazole.
[0054] According to some preferred embodiments of the present invention, trans-(N-Boc-4-aminocyclohexyl)acetic acid and 1-(2,3-dichlorophenyl)piperazine are present in the presence of a condensing agent in step (1). Condensation reaction can be carried out directly to obtain the compound shown in formula 1.
[0055] According to some preferred embodiments of the present invention, the condensing agent is selected from 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, dicyclohexylcarbodiimide and 2 -at least one of (7-benzotriazole oxide)-N,N,N',N'-tetramethylur...
Embodiment 1
[0143] Synthesis of trans N-tert-butoxycarbonyl-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexylamine
[0144]
[0145] Suspend trans-(N-Boc-4-aminocyclohexyl)acetic acid (100g, 0.39mol) in dichloromethane (500ml) and slowly add thionyl chloride (69.5g, 0.59mol) at 7-8°C After the addition, the system was heated to 25° C. and stirred for 4 h to obtain a reaction solution. The reaction solution was concentrated to dryness under reduced pressure, and 300 ml of dichloromethane was added to obtain an acid chloride solution, which was set aside.
[0146] Weigh 1-(2,3-dichlorophenyl)piperazine hydrochloride (125.7g, 0.47mol), add 1.2L water to dissolve, adjust the pH to 7~8 with sodium carbonate, and extract with dichloromethane (500mL *2 times), dried over anhydrous sodium sulfate to obtain a dichloromethane extract, added triethylamine (78.8 g, 0.78 mol) to the dichloromethane extract, and cooled in an ice-water bath. Control the temperature at 15°C and add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com