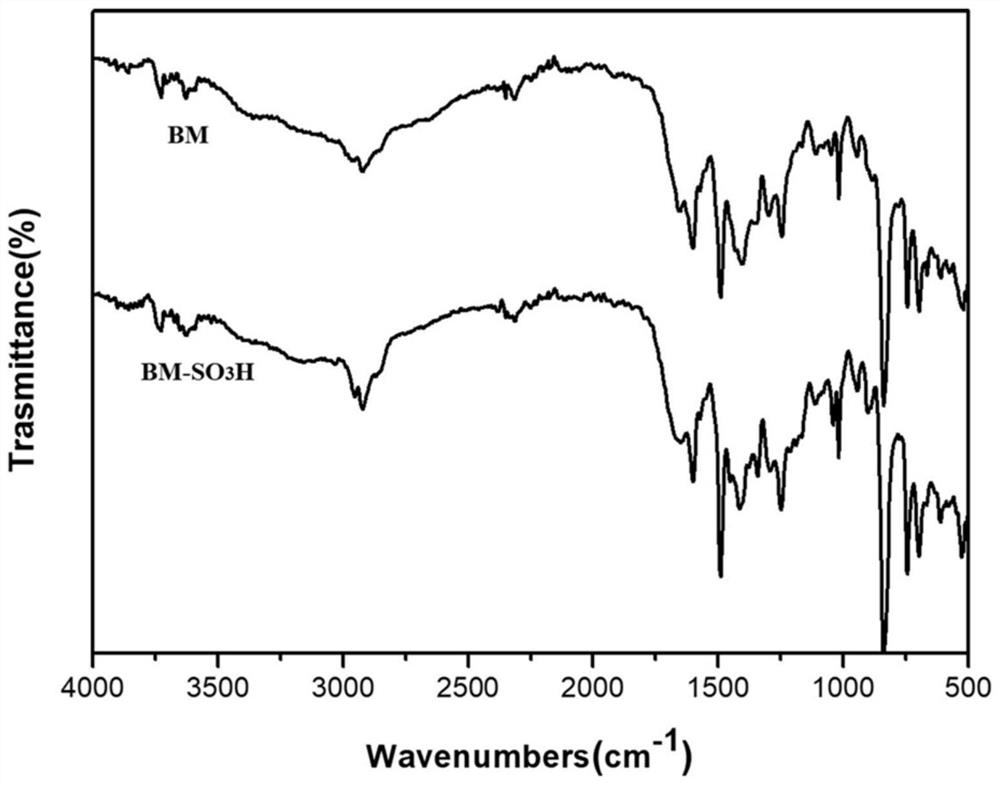
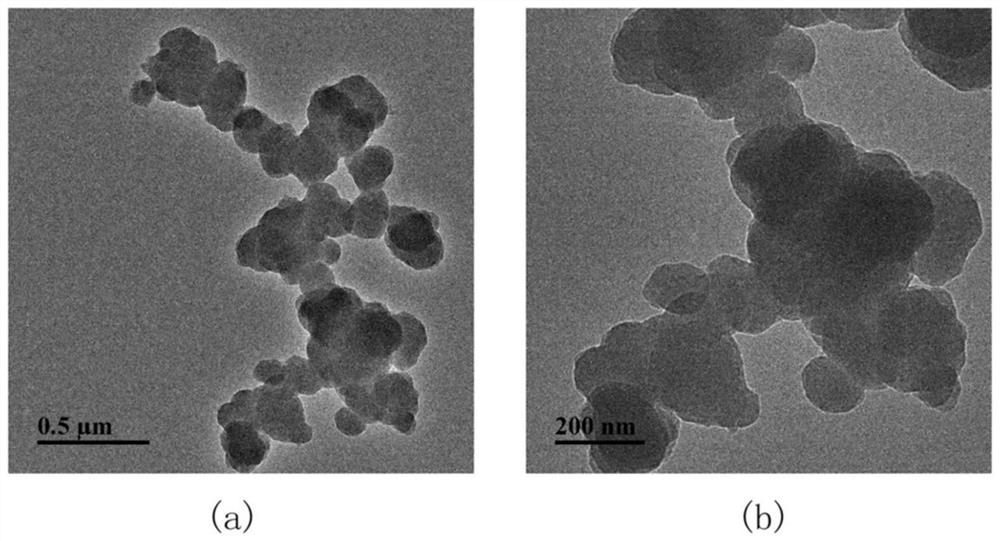
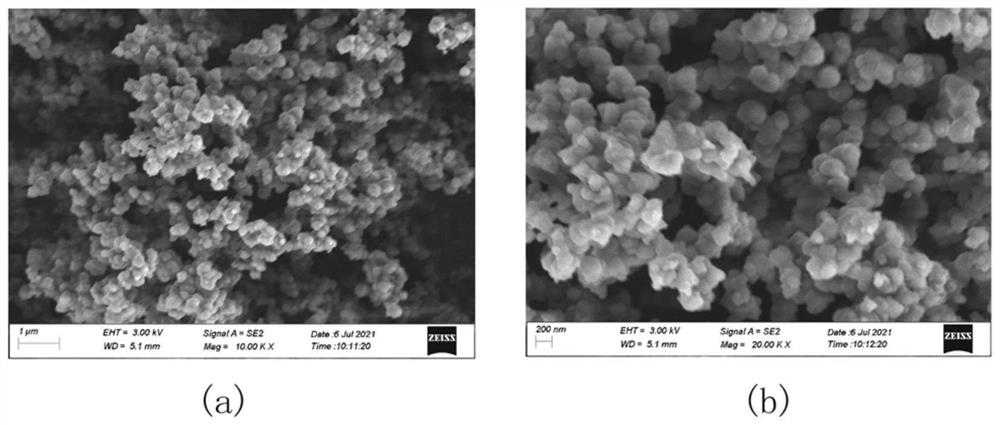
Covalent organic framework material BM-SO3H with acid-base dual functions as well as preparation method and application of covalent organic framework material BM-SO3H
A covalent organic framework, acid-base dual function technology, applied in the preparation of organic compounds, the preparation of carboxylic acid nitrile, organic compounds/hydrides/coordination complex catalysts, etc., to achieve mild catalytic conditions, good thermal stability, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A covalent organic framework material BM-SO with dual functions of acid and base 3 H has a structure as shown in formula (I):
[0034]
[0035] A covalent organic framework material BM-SO with dual functions of acid and base 3 The preparation method of H, concrete steps are as follows:
[0036] (1) Synthesis of 1,3,5-tris(p-formylphenyl)benzene (TFPB)
[0037] Into a 100 mL round bottom flask was added 251 mg of 1,3,5-tribromobenzene, 553 mg of 4-formylphenylboronic acid, 530 mg of potassium carbonate and 10 mg of Pd (pph 3 ) 2 Cl 2 , and then added 40 mL of ethanol, and refluxed at 80° C. for 8 h. After the reaction finishes, remove salt of wormwood and catalyst Pd (pph 3 ) 2 Cl 2 , the filtrate was collected and concentrated, and the trisubstituted product 1,3,5-tri(p-formylphenyl)benzene was obtained as a white solid by column chromatography.
[0038]
[0039] (2) Synthesis of benzimidazole-based covalent organic framework material BM
[0040] Add 100...
Embodiment 2
[0050] The BM-SO that embodiment 1 makes 3 As a heterogeneous catalyst, H participates in the series decarboxylation Knoevenagel condensation reaction, as shown in formula (II), and the specific steps are as follows:
[0051]
[0052] (1) Add respectively benzaldehyde dimethyl acetal (0.152g, 1mmol), malononitrile (0.07g, 1.05mmol), 1mol% BM-SO4 to the reaction flask 3 H (0.005g) and water (5mL) were stirred at 60°C for 5h. After the reaction, separate and reclaim the filter cake by filtration (i.e. catalyst BM-SO 3 H), the filtrate was first extracted with ethyl acetate to obtain an organic layer, and then the product was purified by silica gel column chromatography, the eluent was ethyl acetate and petroleum ether, and the product yield was calculated.
[0053] (2) Catalyst BM-SO recovered in the reaction 3 H was washed with ethanol, centrifuged, vacuum-dried, and then subjected to the next cycle of catalysis under the same conditions. The catalysis reaction was cycled...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com