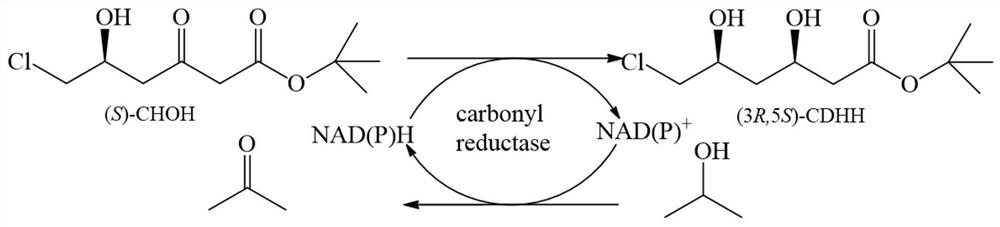
Carbonyl reductase mutant and application thereof in preparation of rosuvastatin chiral intermediate
A reductase and mutant technology, applied in the field of stereoselective carbonyl reductase mutants and their encoding genes, can solve the problem that the specific mechanism has not been accurately explained, and achieves good development and application value, low production cost, and simplification. Effects of chemical catalytic steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of recombinant carbonyl reductase mutant library
[0029] The carbonyl reductase coding gene SEQ ID NO.1 was connected with the expression vector pET28b to construct a heterologous expression recombinant plasmid containing the carbonyl reductase coding gene, and the expression recombinant plasmid was transformed into the host bacteria E. coli In BL21 (DE3), the recombinant genetically engineered bacteria containing the recombinant plasmid were obtained. With recombinant bacteria containing the expression vector pET28b-SCR ( E. coli BL21(DE3) / pET28b-SCR) was used as the starting strain, and the mutation library was constructed by semi-rational design method, and the carbonyl reductase substrate ( S )-CHOH catalytic activity.
[0030] Site-directed saturation mutation:
[0031] Val (V) 153:
[0032] Upstream primer 1: 5'-GGCGATCCGACT NNK GGCGCTTATAAC-3'
[0033] Downstream primer 3: 5'-GTTATAAGCGCC MNN AGTCGGATCGCC-3'
[0034] Gly(G) 23...
Embodiment 2
[0038] Example 2: Screening of recombinant carbonyl reductase mutants
[0039] Randomly pick a single colony from the obtained mutant library for sequencing analysis to obtain different mutant amino acids at different mutation points, and then perform enzyme activity assay analysis to establish the optimal mutation point.
[0040] Screening of positive clones:
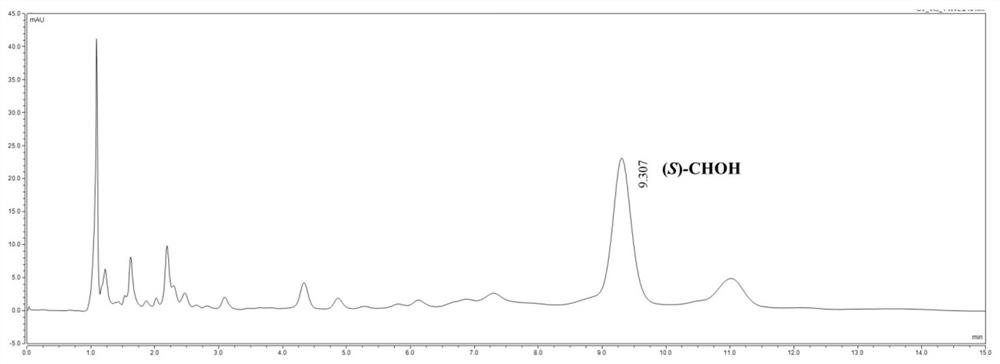
[0041] Through the saturation mutation analysis of Val(V)153 and Gly(G)233 sites, a series of different amino acid mutants were obtained. The optimal mutant was determined by comparing the conversion rate of different mutants when catalyzing the substrate. The transformation reaction was carried out in a 10mL transformation bottle, the concentration of the substrate ((S)-CHOH was 100g / L, isopropanol was 4mL, the mutant wet bacteria was 15g / L, 30°C, 600rpm magnetic stirring for 20min, and the reaction solution was passed through HPLC analysis is used to determine the conversion rate of the reaction to determine the op...
Embodiment 3
[0043] Example 3: Preparation of recombinant carbonyl reductase mutant wet thallus
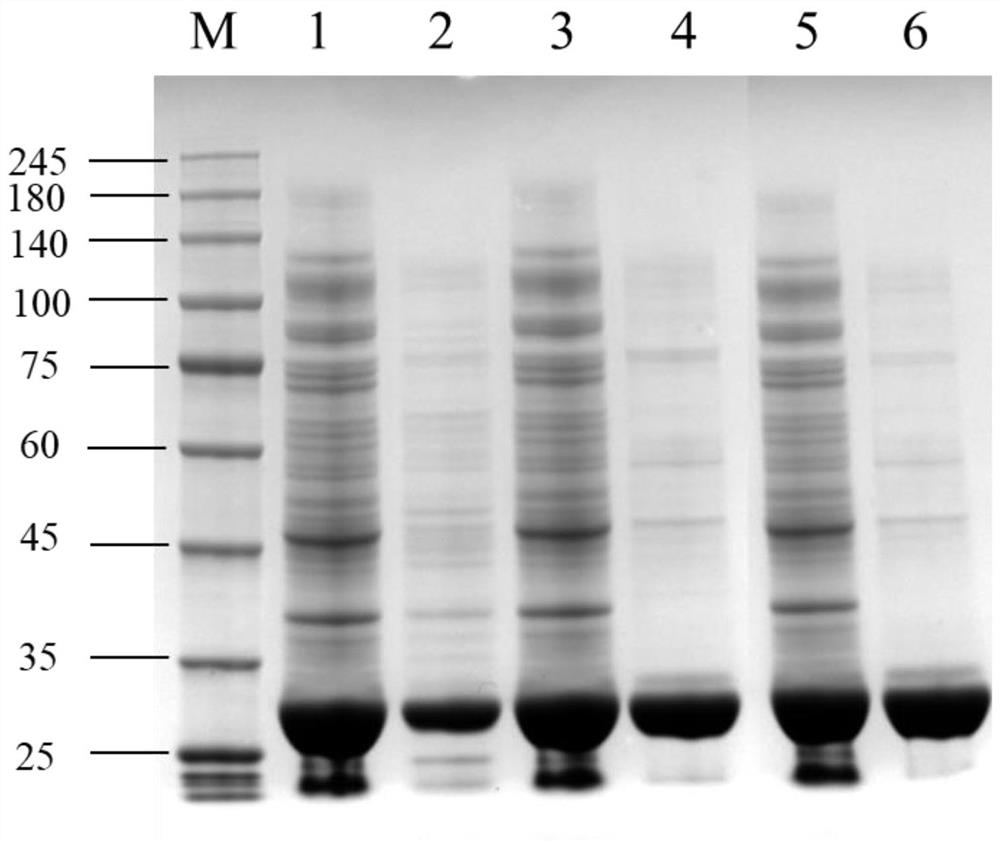
[0044] Inoculate the recombinant Escherichia coli (recombinant Escherichia coli BL21(DE3) / pET28b-mut-Val153Cys, recombinant Escherichia coli BL21(DE3) / pET28b-mut-Gly233Asn) obtained in Example 2 containing the recombinant carbonyl reductase mutant gene into LB liquid medium containing kanamycin resistance at a final concentration of 50 μg / mL, cultivated at 37°C for 8 hours at 200 rpm, and then inoculated with 2% ( v / v ) was inoculated into fresh LB liquid medium containing a final concentration of 50 μg / mL kanamycin resistance, and cultured at 37°C and 200 rpm until the cell OD 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 8000 rpm for 15min at 4°C, discard the supernatant, collect the precipitate, and obtain the mutant gene containing the expression of recombinant carbonyl reductase Recombinant Escherichia coli wet ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com