Method for simultaneously preparing methyl methoxyacetate and methyl glycolate
A technology of methyl methoxyacetate and methyl glycolate, which is applied in the field of simultaneous preparation of methyl methoxyacetate and methyl glycolate, can solve the problems of inorganic acid pollution, difficult separation of products, corrosion, etc., and achieve non-corrosion The effect of reaction equipment, saving reaction cost, and easy separation and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Carbonylation reaction
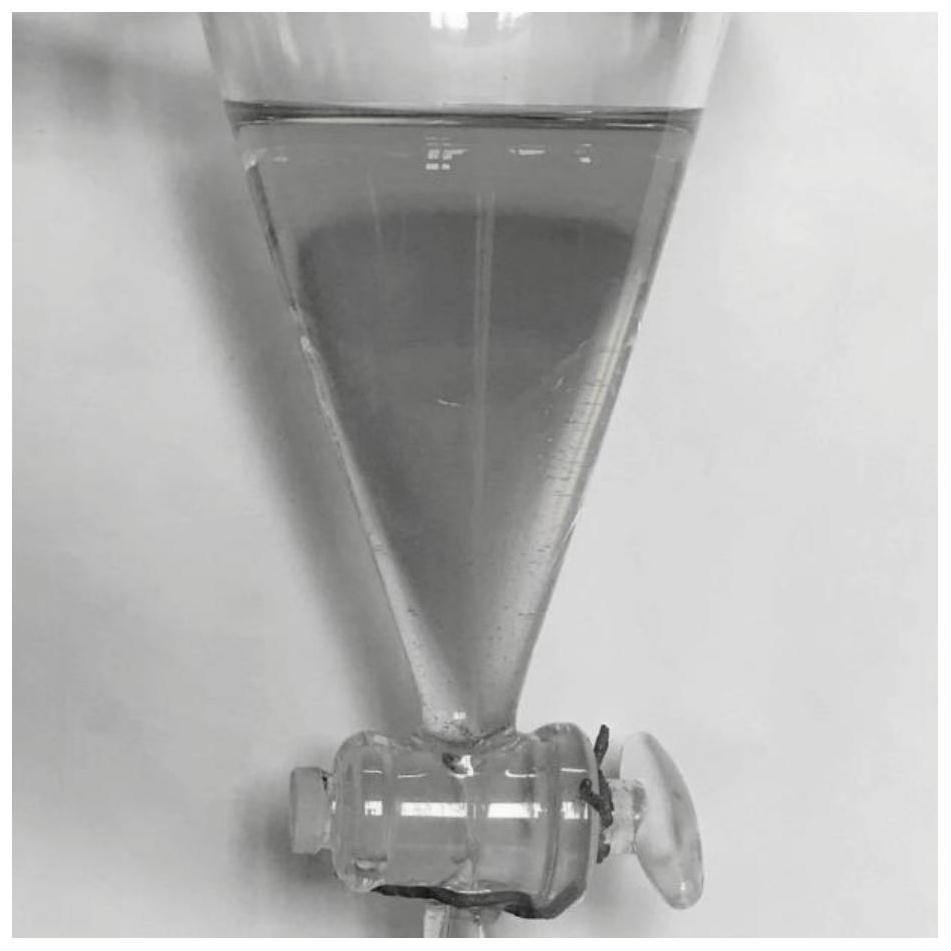
[0031] In a stainless steel autoclave with a volume of 100mL, add 8.1g (0.1mol HCHO) of formaldehyde aqueous solution (Shanghai test, containing about 37% formaldehyde and 10% methanol), cyclohexane:isobutyric acid=5:1 (molar ratio ) mixed solvent 50mL. Quickly weigh 0.185 g (0.2 mmol, CAT / HCHO=0.2%) of tris(triphenylphosphine)rhodium carbonyl hydride and put it into the kettle, stir and mix well, then seal the reaction kettle, replace the air in the kettle with CO for 3 times, Introduce high-pressure CO to 7MPa, and react at 120°C for 3h. After the reaction was over, the reactor was cooled to room temperature, all the reaction solution was taken out and placed in a separatory funnel, and left to stand until the layers were separated, such as figure 1 shown. Filter the dark liquid in the lower layer through a microporous membrane to obtain a light brown-yellow carbonylation product, which is set aside.
[0032] 2. Esterification reaction ...
Embodiment 2
[0036] 1. Recovery of reaction solvent
[0037] The upper layer of the liquid after the carbonylation reaction in Example 1 was taken out, filtered through a microporous membrane, and set aside.
[0038] 2. Carbonylation reaction
[0039] Put the filtered upper solvent into a 100mL stainless steel autoclave, add 8.1g (0.1mol formaldehyde) of formaldehyde aqueous solution (Shanghai test, containing about 37% formaldehyde and 10% methanol), and add 8mL of isobutyric acid. Quickly weigh 0.185 g (0.2 mmol, CAT / HCHO=0.2%) of tris(triphenylphosphine)rhodium carbonyl hydride and put it into the kettle, stir and mix well, then seal the reaction kettle, replace the air in the kettle with CO for 3 times, Introduce high-pressure CO to 7MPa, and react at 120°C for 3h. After the reaction, the reactor was cooled to room temperature, all the reaction solution was taken out and placed in a separatory funnel, and allowed to stand until the layers were separated. Filter the dark liquid in th...
Embodiment 3
[0044] 1. Carbonylation reaction
[0045] In a stainless steel autoclave with a volume of 100mL, add 5.5g (containing 0.1mol formaldehyde) of formaldehyde aqueous solution (Shanghai test, containing about 55% formaldehyde and 15% methanol), n-octane:isobutyric acid=2:1 (mol than) mixed solvent 50mL. Quickly weigh 0.196 g (0.4 mmol, CAT / HCHO=0.4%) of triphenylphosphinecarbonyl rhodium acetylacetonate into the kettle, stir and mix well, then seal the reaction kettle, replace the air in the kettle with CO for 3 times, and inject High pressure CO to 7MPa, react at 120°C for 3h. After the reaction, the reactor was cooled to room temperature, all the reaction solution was taken out and placed in a separatory funnel, and allowed to stand until layers were separated. Filter the lower layer liquid through a microporous membrane to obtain the carbonylation product, which is set aside.
[0046] 2. Esterification reaction
[0047] Put the filtered carbonylation product of the lower la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com