Synthesis method of saflufenacil key intermediate
A technology of saflufenacil and a synthesis method, applied in the field of carbamate synthesis, can solve problems to be developed and the like, achieve the effects of high product yield, control of chlorinated isomers, and improved product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
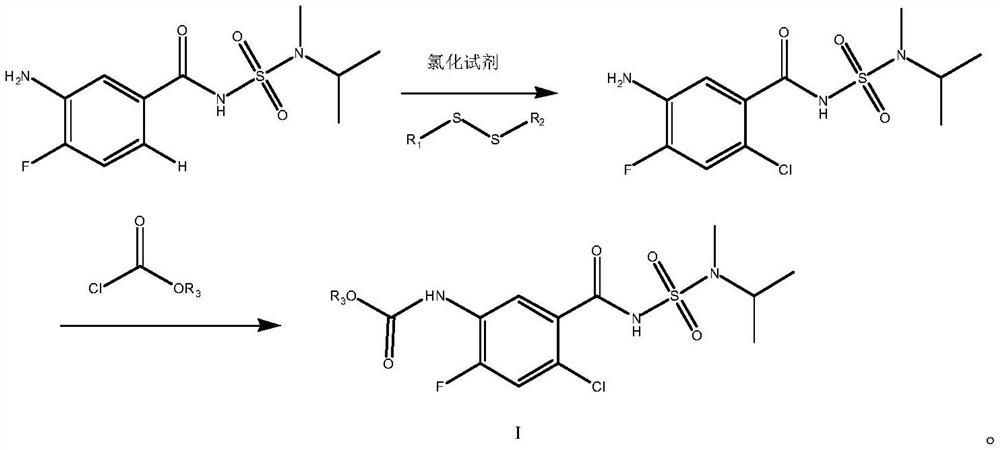
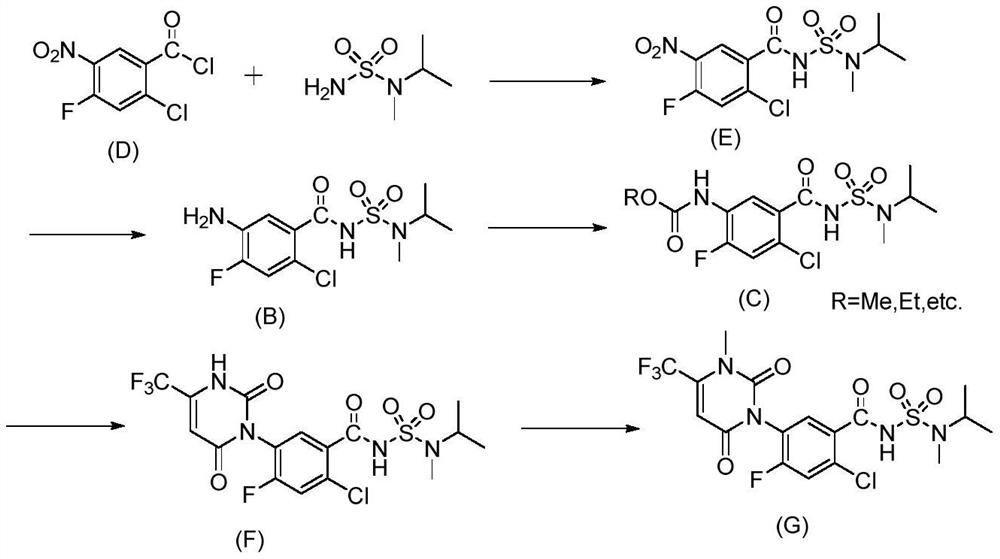
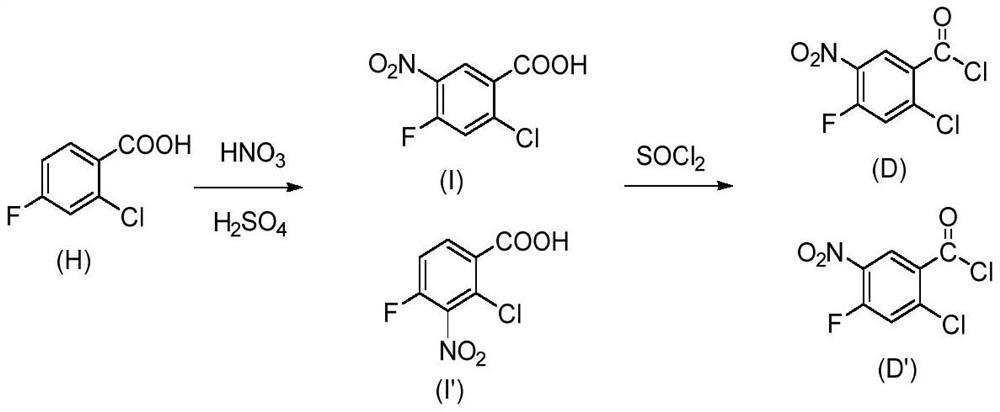
[0040] Embodiment 1: the synthesis of selective chlorination reaction raw material
[0041](1) Synthesis of N-(3-nitro-4-fluorobenzoyl)-N’-methyl-N’-isopropylsulfonamide
[0042]
[0043] In 60ml of toluene, add N-methyl-N-isopropylaminosulfonamide (16.74g, 0.11mol), catalyst dimethylaminopyridine (72.0mg, 0.6mmol), acid-binding agent triethylamine (24.5g, 0.24mol), stirred and dissolved, then heated to 70°C, under nitrogen atmosphere, added the toluene solution of 3-nitro-4-fluorobenzoyl chloride (20.4g, 0.10mol) dropwise to the reaction system, added dropwise for 1h, after the dropwise addition was completed The suspension was cooled to room temperature and stirring was continued for 2 hours. The mixture was acidified by adding concentrated hydrochloric acid and stirred for 1 hour, the precipitated salt was filtered, washed once with 1N HCl solution, and the resulting wet solid was recrystallized by adding 50 g of chlorobenzene. Finally filter and dry under reduced pres...
Embodiment 2
[0047] Example 2: Synthesis of N-(5-amino-2-chloro-4-fluorobenzoyl)-N'-methyl-N'-isopropylsulfonamide
[0048]
[0049] Add N-(4-fluoro-3-aminobenzoyl)-N'-methyl-N'-isopropylsulfonamide (2.89g, 10mmol, 1.0eq), N-chlorobutanedi Imide (4.05g, 30mmol, 3.0eq) was dissolved in 100mL acetonitrile and stirred for 30min; dimethyl disulfide (1.22g, 13.0mmol, 1.3eq) was added at room temperature 25°C under nitrogen atmosphere. After the generated product was stirred and reacted at room temperature under a nitrogen atmosphere for 0.5-1h (monitored by LC), the reaction was basically converted, and concentrated by precipitation under negative pressure. The residual liquid was subjected to column chromatography (PE:EA=1:2) to obtain 2.90 g of the target compound at ~162°C, the product is a light yellow solid, the yield is 87%, and the HPLC purity is 97%.
Embodiment 3
[0050] Example 3: Synthesis of N-(5-amino-2-chloro-4-fluorobenzoyl)-N'-methyl-N'-isopropylsulfonamide
[0051] Add N-(4-fluoro-3-aminobenzoyl)-N'-methyl-N'-isopropylsulfonamide (2.89g, 10mmol, 1.0eq), N-chlorobutanedi Imide (4.05g, 30mmol, 3.0eq) was dissolved in 100mL dichloromethane and stirred for 30min; dimethyl disulfide (1.22g, 13.0mmol, 1.3eq) was added at room temperature 25°C under nitrogen atmosphere. After the generated product was stirred and reacted at room temperature under a nitrogen atmosphere for 0.5-1h (monitored by LC), the reaction was basically converted, and concentrated by precipitation under negative pressure. The residual liquid was subjected to column chromatography (PE:EA=1:2) to obtain 2.87g of the target compound at ~162°C, the product is a light yellow solid, the yield is 85%, and the HPLC purity is 96%. 1 H-NMR (400MHz, DMSO-d 6 )δ11.91(br s,1H),7.26(d,J=8.0Hz,1H),6.85(d,J=8.0Hz,1H),5.60(s,2H),4.10-4.06(m,1H) , 2.81 (s, 3H), 1.12 (d, J=8.0Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com