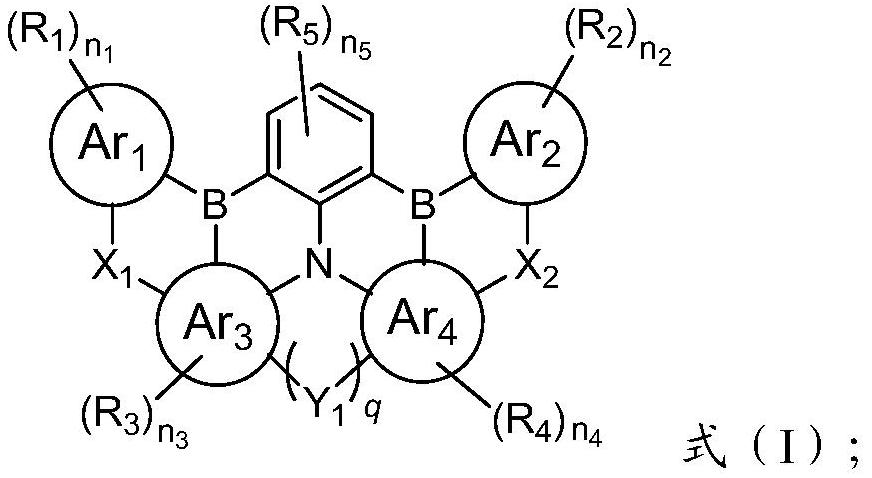
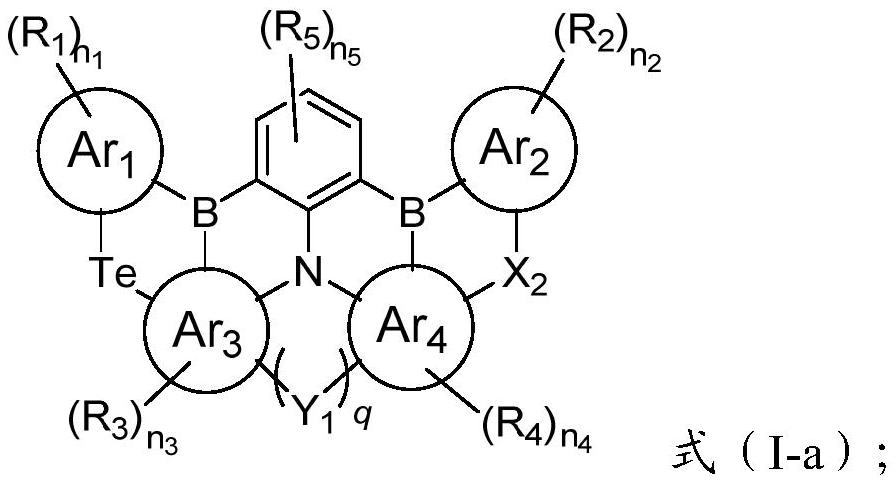
Condensed ring compound containing boron atom, nitrogen atom and selenium atom or tellurium atom and organic electroluminescent device
A nitrogen atom and boron atom technology is applied in the field of fused ring compounds and organic electroluminescence devices, which can solve the problems of complex device structure and reduced external quantum efficiency of the device, and achieve high luminous efficiency, narrow electroluminescence half-peak width, The effect of high device external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The present invention has no special limitation on the preparation method of the condensed ring compound, and a typical preparation process thereof is as follows:
[0051] After the compound shown in formula (II) is reacted with alkyllithium, then reacted with boron trihalide and organic amine to obtain the condensed ring compound shown in formula (I); said alkyllithium is preferably butyllithium, secondary One or more of butyllithium, tert-butyllithium, methyllithium and ethyllithium; the boron trihalide is preferably boron trifluoride, boron trichloride, boron tribromide and boron triiodide One or more of them; the organic amine is preferably one or more of N, N-diisopropylethylamine, triethylamine and tri-n-butylamine;
[0052]
[0053] Lu 1 It is halogen; other code names are the same as above, and will not be repeated here.
[0054] Alternatively, a typical preparation process thereof is as follows:
[0055] The compound shown in formula (III) is reacted with...
Embodiment 1
[0075] The reaction formula is as follows:
[0076]
[0077] Add 1-1(2-bromo-1-fluoro-3-iodobenzene) (79.4g, 264.0mmol), aniline (11.2 g, 120.0mmol), Pd 2 (dba) 3 (3.3g, 3.6mmol), t-Bu 3 P·BF 4 (4.18g, 14.4mmol) and sodium tert-butoxide (26.0g, 270.0mmol), take 500mL of toluene and add it to the bottle, heat up to 110°C, stir the reaction for 24 hours under the protection of argon, then cool to room temperature, at 50°C After the toluene was removed under reduced pressure, the reaction solution was washed with deionized water, extracted with ether to separate the organic phase, and then dried by adding anhydrous sodium sulfate. The organic phase was filtered to remove the solvent, and the crude product was separated by silica gel column to obtain product 1-2 (44.7 g, yield: 85%). Elemental analysis of its structure (C 18 h 11 Br 2 f 2 N): theoretical value C, 49.24; H, 2.53; N, 3.19; test value C, 49.31; H, 2.50; N, 3.23. MALDI-TOF MS analysis: theoretical value 43...
Embodiment 2
[0083] The reaction formula is as follows:
[0084]
[0085] 2-1 (2,7-dibromo-9-phenylcarbazole) (28.8g, 72.0mmol), 4-tert-butylphenylselenol (12.84g, 60.0mmol), Pd 2 (dba) 3 (1.37g, 1.5mmol), Xantphos (1.74g, 3.0mmol) and N,N-diisopropylethylamine (15.5g, 20.9mL, 120.0mmol), add 300mL 1,4-dioxane to the bottle , heated to reflux, stirred and reacted for 12 hours under the protection of argon, then cooled to room temperature, poured the reaction solution into 1.2L deionized water, stirred for 1 hour, extracted with dichloromethane to separate the organic phase, and then added anhydrous sulfuric acid After drying over sodium, the organic phase obtained by filtration was removed from the solvent, and the crude product was separated through a silica gel column to obtain product 2-2 (23.0 g, yield: 72%). Elemental analysis of its structure (C 28 h 24 BrNSe): theoretical value C, 63.05; H, 4.54; N, 2.63; found value C, 63.15; H, 4.50; N, 2.65. MALDI-TOF MS analysis: theoret...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com