Sulfonyl-containing polysubstituted aromatic diamine monomer and preparation method thereof
An aromatic diamine and multi-substitution technology, which is applied in the field of diamine monomer preparation, can solve the problems of single polyimide functional group type, polyimide film is easy to break, polyimide development restrictions, etc., to achieve good solubility The effect of safety, cost saving and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
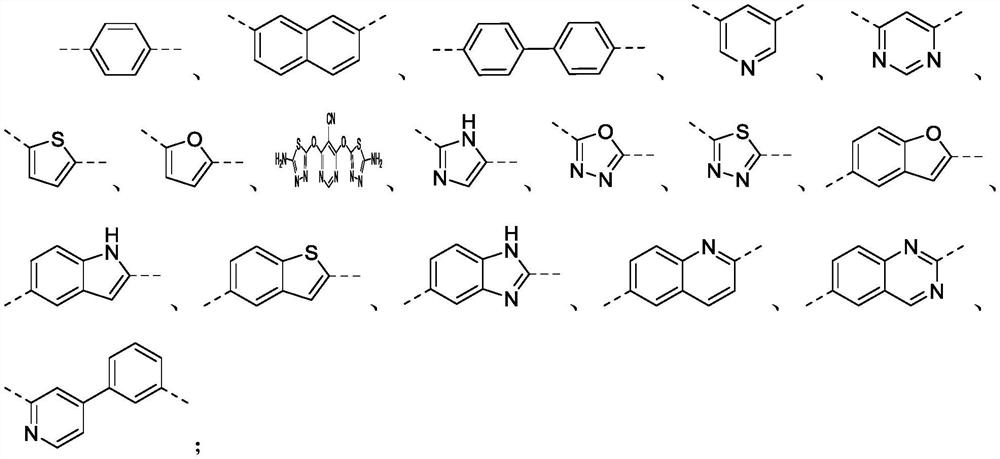
[0076] (1) under the protection of nitrogen, into the three-necked flask equipped with mechanical stirring, add the molar ratio of 2.0:1.0 with substituent R 1 The obtained p-aminophenol A and m-dihalobenzenesulfonyl compound B were further added with an appropriate amount of organic solvent and basic catalyst, stirred at room temperature for half an hour, and then heated to 70 °C for 7 hours to complete the reaction.
[0077] (2) A white aromatic diamine monomer can be obtained after precipitation, filtration, drying and recrystallization.
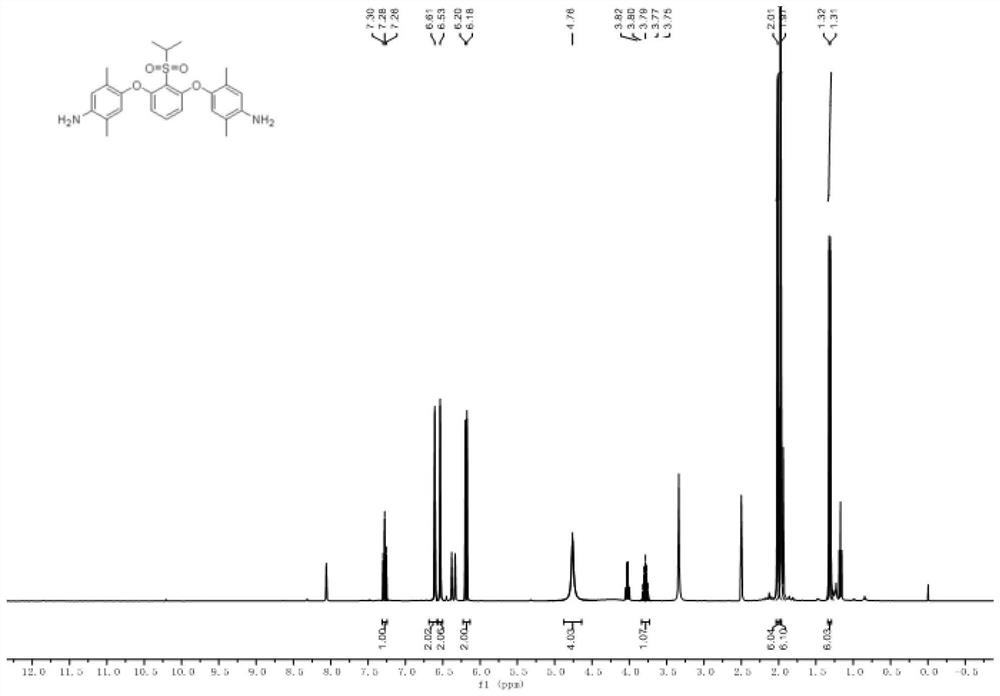
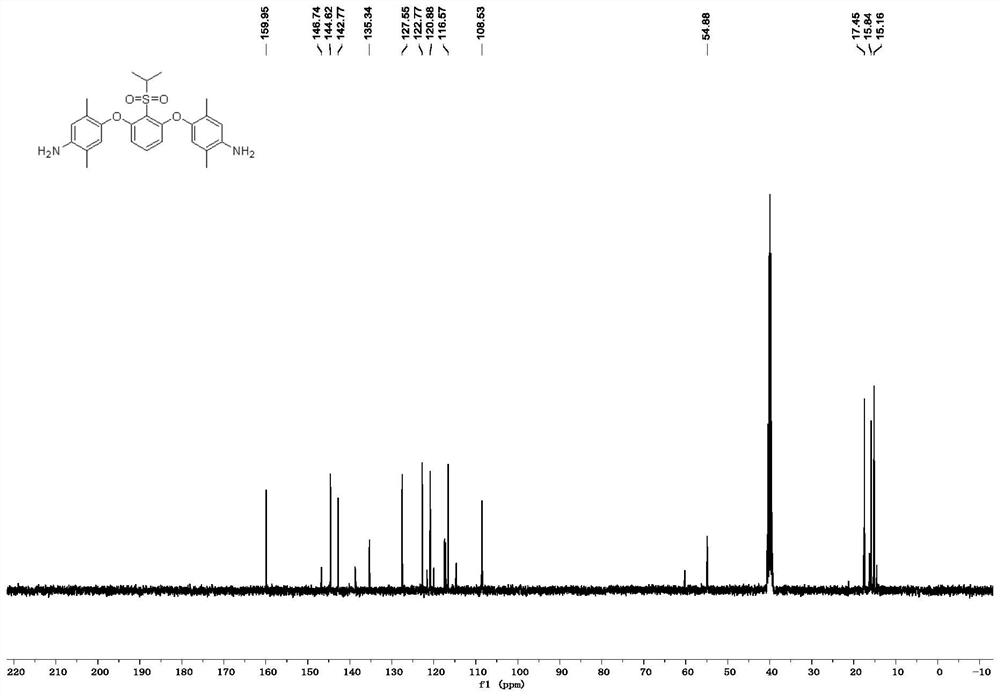
[0078] The specific results are Figure 1-2 shown.
[0079] Wherein, the organic solvent selected in this embodiment is a mixture of 1,4-dioxane and N,N-dimethylformamide in a mass ratio of 4:6, and the amount thereof is the same as that of the p-amino group. The mass ratio of the amount and the amount of the phenol and the m-dihalobenzenesulfonyl compound is 3.0: 1.0; the basic catalyst is selected as potassium hydroxide, and the mass ...
Embodiment 2-9
[0085] The specific embodiment is the same as Example 1, the difference is that the reaction temperature, reaction time and the molar ratio of the raw materials will be somewhat different. The specific setting parameters and the purity of the target product are shown in Table 1.
Embodiment 10
[0087] (1) Under the protection of ammonia gas, in a three-necked flask equipped with mechanical stirring, add a molar ratio of 3.0:1.0 with substituent R 1 The p-aminophenol A and the m-dihalobenzenesulfonyl compound B were further added with an ionic liquid catalyst, stirred at room temperature for half an hour, heated to 80 °C and reacted for 10 hours, and the reaction was terminated.
[0088] (2) The product obtained by the reaction is centrifuged, and the solid obtained by centrifugation is washed, dried and recrystallized to obtain a white aromatic diamine monomer.
[0089] Wherein, the rotating speed of centrifugation is 5000rpm, the time of centrifugation is 30min, and the selected ionic catalyst is ionic catalyst 1, and the structural formula of described ionic catalyst 1 is The mass ratio of its consumption to the consumption of the p-aminophenol and the m-dihalobenzenesulfonyl compound is 3.0:1.0; the alcohol used during recrystallization is methanol; Ar 1 Select ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com