Preparation method of cariprazine intermediate
An intermediate and reaction technology, applied in the field of preparation of cariprazine intermediates, can solve the problems of incomplete reaction, unstable temperature, process impurities and the like, and achieve the effects of few steps, easy availability of raw materials and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
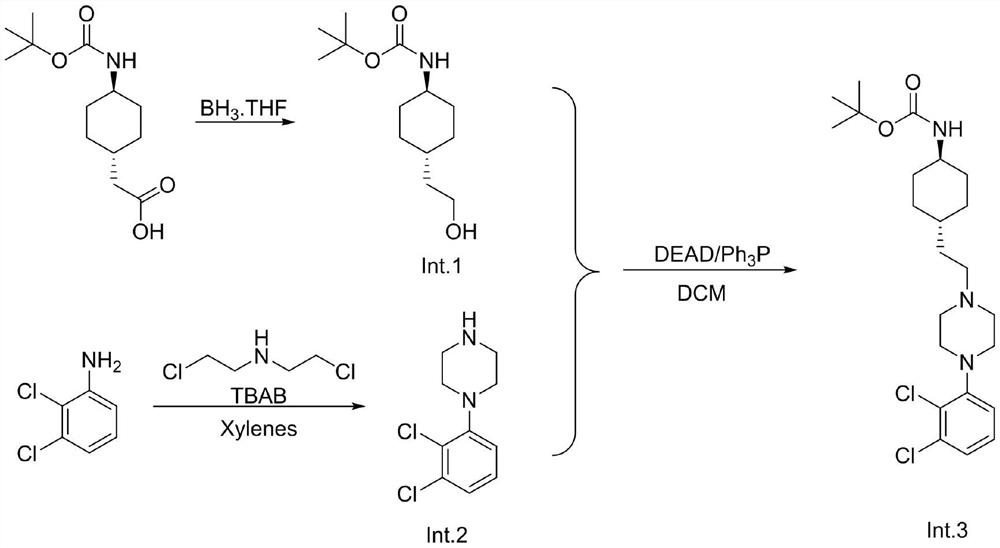
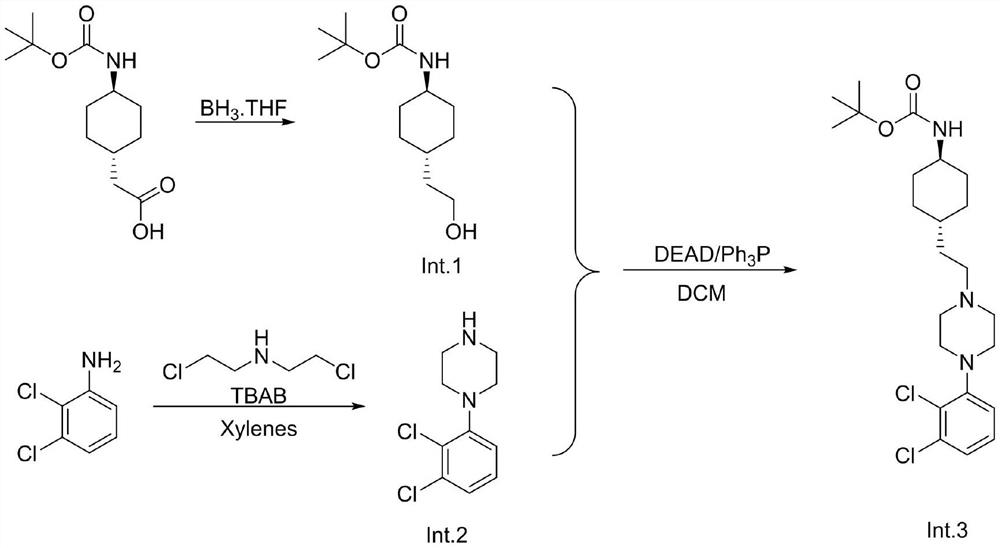
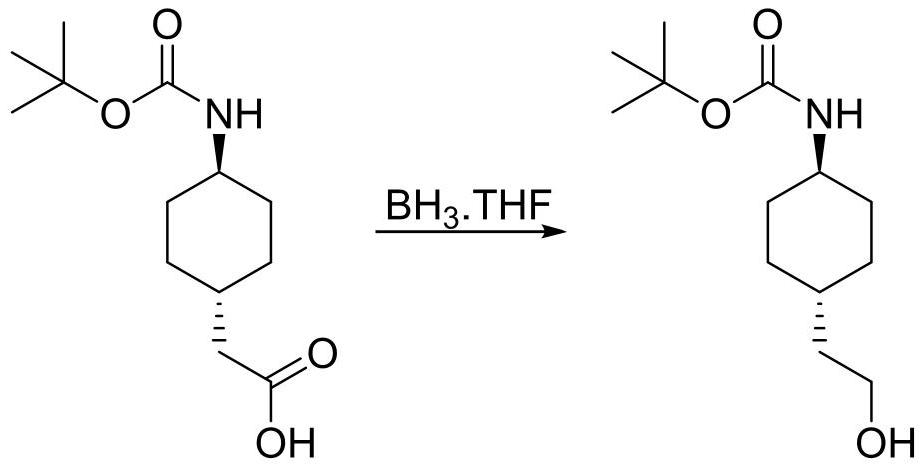
[0024] Trans-(N-Boc-4-aminocyclohexyl)acetic acid (25.7g, 0.1mol) was added to the reaction flask, dissolved in 300mL of tetrahydrofuran, the temperature was lowered to 0°C, and 1.0M / L borane tetrahydrofuran solution was added dropwise 150 mL (0.15 mol), and reacted at this temperature for 3 hours. Water was added to quench, MTBE was added, washed twice with saturated aqueous sodium bicarbonate solution, and the organic phase was concentrated to no flow to obtain trans-(N-Boc-4-aminocyclohexyl)ethanol 22.5g, HPLC 96%, yield 92%.
Embodiment 2
[0026]
[0027] To the reaction flask was added 2,3-dichloroaniline 1 (10g, 61.72mmol, 1eq.) and bis(2-chloroethyl)ethylamine 1 (8.7g, 1eq, 61.72mmol) The stirring solution was added p-toluene A solution of sulfonic acid (1.17 g, 0.1 eq, 6.17 mmol) and tetrabutylammonium bromide (1.5 g, 0.1 eq, 6.17 mmol) in xylene (150 mL). The resulting reaction mixture was heated at 130-135°C for 48 hours. After the reaction is complete. The reaction mixture was cooled to room temperature and the pH of the solution was adjusted to pH 6-7 with aqueous ammonia. The organic compound was extracted with ethyl acetate, dried over sodium sulfate, and dried under reduced pressure. After concentration, 12.5 g of 1-(2,3-dichlorophenyl)piperazine was obtained, HPLC 91.6%, yield 88%. Go to the next step without purification.
Embodiment 3
[0029]
[0030] Under nitrogen protection, 24.3g (0.1mol) of trans-(N-Boc-4-aminocyclohexyl)ethanol (Int.1), 1-(2,3-dichlorophenyl)piperazine were added to the reaction flask. (Int.2) 25.4 g (0.11 mol), 31.5 (0.12 mol) of triphenylphosphine and 240 mL of dried dichloromethane, under nitrogen atmosphere, ice-bath temperature-controlled 0-5 ℃ diethyl azodicarboxylate was added dropwise 210 mL of 22.6 g (0.123 mol) of dichloromethane was dripped and kept the temperature stirring for 3 hours. HPLC monitored the reaction and added saturated aqueous sodium bicarbonate solution. The organic phase was concentrated, filtered under reduced pressure, and the filter cake was washed with 20 mL of dichloromethane. Once, a large amount of triphenylphosphine was added to the filter cake, zinc chloride was added to the filtrate, filtered, the filtrate was concentrated, and n-heptane was added to make a slurry to obtain trans-N-tert-butoxycarbonyl-4-{2-[4-(2,3 -Dichlorophenyl)-piperazin-1-yl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com