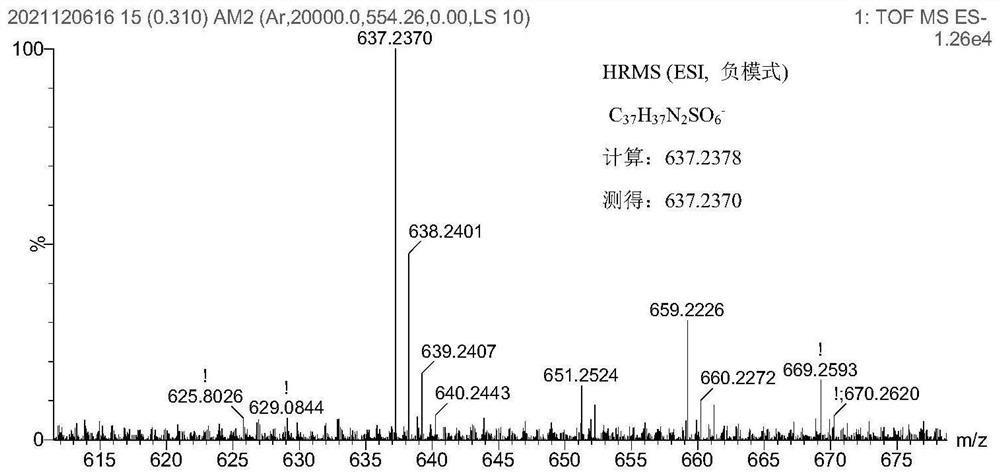
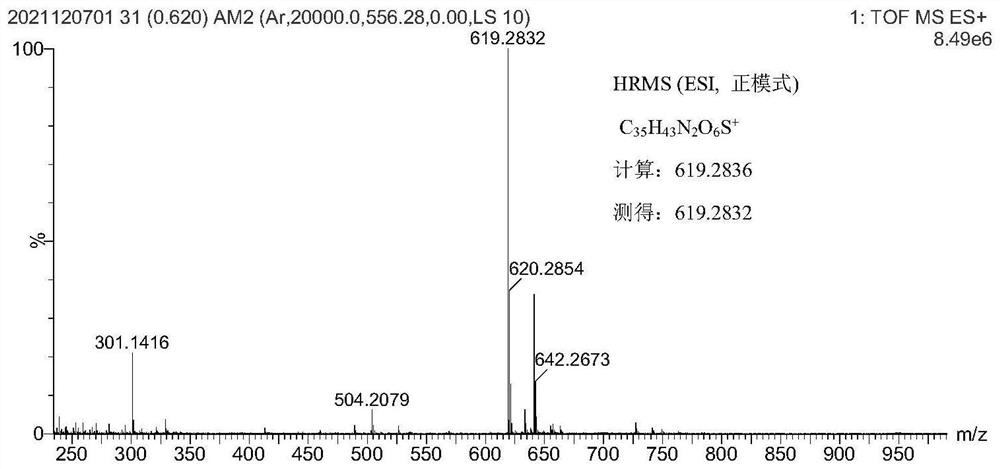
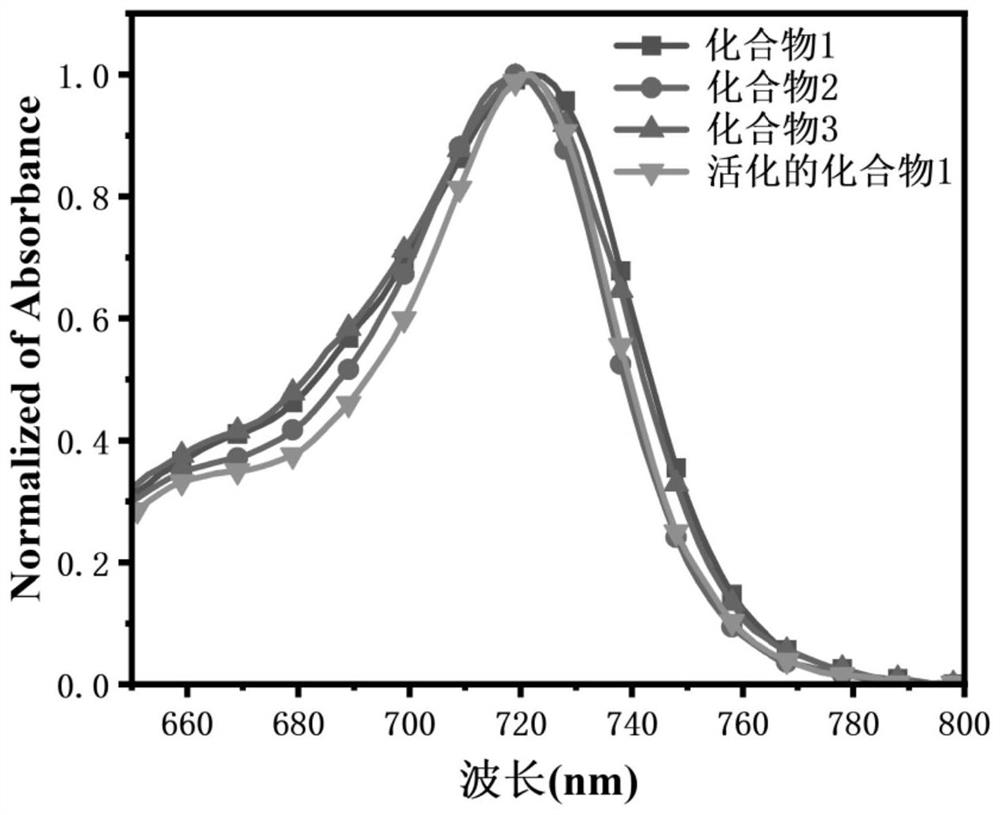
Xanthene-hemicyanine near-infrared fluorescent dye as well as synthesis method and application thereof
A technology of fluorescent dyes and synthesis methods, applied in the field of its synthesis, xanthene-semicyanine near-infrared fluorescent dyes, can solve the problems of fluorescence quenching, molar extinction coefficient, low fluorescence quantum yield, and poor photostability. Achieve good water solubility, increase molar extinction coefficient and fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Production of Compound 1
[0057] The structural formula of compound 1:
[0058]
Embodiment 11
[0060]
[0061] 9 mL of phosphorus tribromide was slowly added dropwise to a mixed solution of 12 mL of DMF and 25 mL of chloroform at 0°C, stirred for 1 h, then warmed to room temperature, added with 4 mL of cyclohexanone, and the reaction was stopped after stirring for 12 h. The solution was poured into 100 mL of ice water, and NaHCO was added 3 Make the solution neutral with CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated to give the title compound 1.1 (6.0 g, 31.74 mmol, Y=73.8%).
Embodiment 12
[0063]
[0064] At room temperature, the compound of Example 1.1 (2.93g, 15.5mmol, 1.0eq) and 4-diethylaminosalicylaldehyde (3.0g, 15.5mmol, 1.0eq) were added to 15mL of DMF, stirred for 16h and then stopped Reaction, pour the solution into 100 mL of water, use CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 The organic phase was dried, concentrated, and purified by silica gel column to obtain yellow-brown compound 1.2 (1.4 g, 4.94 mmol, Y=31.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com