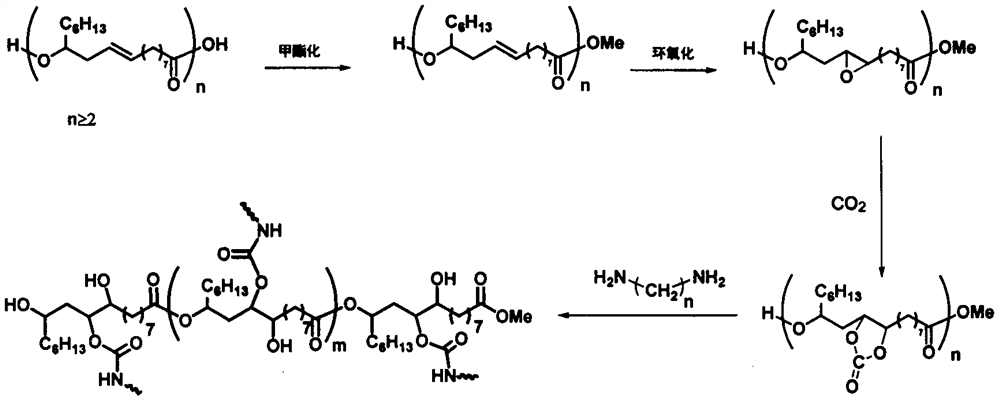
Novel castor-based polyurethane coating material and preparation method thereof
A polyurethane coating and castor-based technology, which is applied in the field of new castor-based polyurethane coating materials and its preparation, can solve the problems of limited production of castor-based polyurethane and achieve the effect of convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening of reaction conditions for methylation of polyricinoleic acid
[0033] Using polyricinoleic acid with an acid value of 60 mg KOH / g as raw material, methyl esterification was carried out, and the optimum conditions for methyl esterification were discussed. Specific steps are as follows:
[0034]Add 10 g of polyricinoleic acid with an acid value of 60 mg KOH / g, 20 to 50 mL of methanol and 0.5 to 3 mL of boron trifluoride-methanol solution into a 50 mL flask. After refluxing for 6-16 h, methanol was removed by rotary evaporator. After adding 30 mL of diethyl ether to dilute, the mixture was transferred to a separatory funnel and washed with water 3 times. The solvent in the organic phase was removed to finally obtain a yellow oily liquid polymethyl ricinoleate.
[0035] Table 1 Screening of reaction conditions for methylation of polyricinoleic acid
[0036]
[0037]
[0038] After screening the conditions, the optimal conditions for the No. 2 ...
Embodiment 2
[0039] Example 2 Screening of reaction conditions for epoxidation of methyl polyricinoleate
[0040] Taking the polyricinoleic acid methyl ester in Example 1 as a raw material, epoxidation was carried out, and the optimum conditions of epoxidation were explored. Specific steps are as follows:
[0041] Disperse 8.6 g of methyl polyricinoleate uniformly in 40-60 mL of diethyl ether, slowly add 5-8 g of m-chloroperoxybenzoic acid, and stir the reaction at room temperature for 12-36 h. Wash with 30 mL of saturated sodium thiosulfate solution, 30 mL of saturated sodium bicarbonate solution and 30 mL of saturated sodium chloride solution successively 3 times. Finally, the ether in the organic phase was removed to obtain a colorless oily liquid epoxidized methyl polyricinoleate.
[0042] Table 2 Screening of reaction conditions for epoxidation of methyl polyricinoleate
[0043]
[0044] After screening of conditions, the No. 3 reaction conditions were selected as the optimal co...
Embodiment 3
[0045] Example 3 Polyepoxidized methyl ricinoleate and CO 2 Screening of cycloaddition reaction conditions
[0046] 7.05g of epoxidized polymethyl ricinoleate obtained in Example 2 was dissolved in 30~40mL DMF and transferred to the autoclave, then 0.14~0.35g (0.66mmol) of tetrabutylammonium bromide was added, and the Carbon dioxide to 2~5MPa, react at 120~140℃ for 18~36h. After the reaction, the catalyst was washed with water to remove the catalyst, and the solvent in the organic phase was removed to obtain a yellow-brown oily liquid, namely polycyclocarbonate methyl ricinoleate.
[0047] Table 3 Polyepoxidized methyl ricinoleate and CO 2 Screening of cycloaddition reaction conditions
[0048]
[0049] After screening the conditions, the conditions for the No. 2 reaction were selected as the best, namely: the amount of catalyst tetrabutylammonium bromide was 3% of the mass of the substrate polyepoxy ricinoleate, and the amount of solvent DMF was 4.2 mL / g polycyclic Met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com