Production process for reducing impurity dichlorobenzene in chlorobenzene
A production process, a technology for chlorinating benzene, applied in the directions of organic chemistry, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of reducing the reaction efficiency, the iron ring is easily corroded, and the chlorine gas is consumed, so as to improve the reaction efficiency and catalysis Efficient effect and stable bonding method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of modified mesoporous silica:
[0031] A1. Add 0.6g of sodium N-laurate sarcosinate to 70mL of deionized water, then add 14mL of 0.1M hydrochloric acid solution, stir for 1 hour, then add 2mL of ethyl orthosilicate, stir at 600r / min for 30min, and age at room temperature 2h, then poured into the reaction kettle, placed in an oven at 80°C for 24h, then centrifuged, washed the precipitate with deionized water, and vacuum-dried to obtain dry powder; then the dry powder was dispersed in ethanol and ethanolamine with a molar ratio of 3.3 / 1 In the mixed solution of , refluxed for 12h, centrifuged, washed several times with 70mL of ethanol, and vacuum-dried for 24h to obtain mesoporous silica. The dosage ratio of the dry powder to the mixed solution of ethanol and ethanolamine was 1:15;
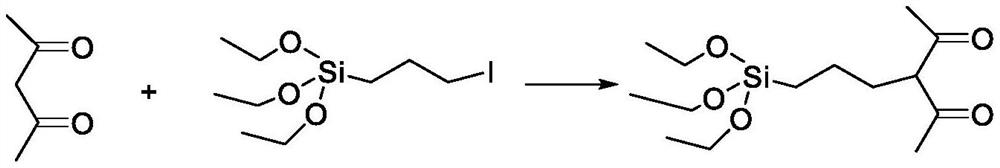
[0032] A2. Add 0.01mol of potassium to 8mL of dry tert-butanol, stir until the potassium is completely dissolved, then slowly add 0.01mol of acetylacetone dropwise at room temper...
Embodiment 2
[0035] Preparation of modified mesoporous silica:
[0036]A1. Add 0.65g of sodium N-laurate sarcosinate to 90mL of deionized water, then add 17mL of 0.1M hydrochloric acid solution, stir for 1h, then add 3mL of ethyl orthosilicate, stir at 600r / min for 60min, and store at room temperature 2 hours, then poured into the reaction kettle, placed in an oven at 80°C for 24 hours, centrifuged again, washed the precipitate with deionized water, and dried in vacuum to obtain dry powder; then the dry powder was dispersed in a molar ratio of 3.3 / 1 ethanol and In the mixed solution of ethanolamine, reflux for 12 hours, centrifuge, wash with 70 mL of ethanol for several times, and vacuum dry for 24 hours to obtain mesoporous silica. The dosage ratio of the dry powder to the mixed solution of ethanol and ethanolamine is 1:20;
[0037] A2. Add 0.01 mol potassium to 15 mL of dry tert-butanol, stir until the potassium is completely dissolved, then slowly add 0.01 mol of acetylacetone dropwise ...
Embodiment 3
[0040] A production technique for reducing impurity dichlorobenzene in chlorinated benzene, comprising the following steps:
[0041] Step 1. Put the modified mesoporous silica prepared in Example 1 into the ferric chloride solution, stir at room temperature for 1.5 hours, stand for 12 hours, centrifuge, wash several times with deionized water, filter again, and precipitate in drying in a vacuum oven to obtain a supported catalyst, wherein the solid-to-liquid ratio of the modified mesoporous silica and the ferric chloride solution is 1g:15mL, and the mass concentration of the ferric chloride solution is 10%;
[0042] Step 2, adding the supported catalyst into the reactor, then adding benzene, feeding chlorine gas, using self-priming stirring to distribute the chlorine gas in the benzene, controlling the reaction temperature to be 78°C, and after reacting for 90 minutes, release a chlorinated liquid containing benzene. Hydrogen chloride gas is discharged from the top of the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com