HPLC (High Performance Liquid Chromatography) fingerprint spectrum construction method and detection method of Yaohuahuangboo prescription
A technology of Yazuo Niu Ha Zhanbo and fingerprints, applied in the field of chemical detection, can solve the problems of slow development, lack of modern controllable quality control indicators for Yazuo Niu Ha Zhanbo formula, and the quality standard needs to be improved and perfected. Achieve high application value and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] 1) Preparation of the test solution: after dissolving the Yajun Niuha Zhanbo granule powder with an organic solvent, ultrasonic extraction, and filtration, the test solution A is obtained;
[0033] 2) Preparation of reference solution:
[0034] Taking the six main active ingredients of Yajun Niuha Zhanbo Formula, namely Danshensu, protocatechuic acid, protocatechuic aldehyde, p-coumaric acid, rosmarinic acid and salvianolic acid B as the reference substance, use 40-70 % methanol is used as solvent to prepare mixed reference solution of different mass concentrations;
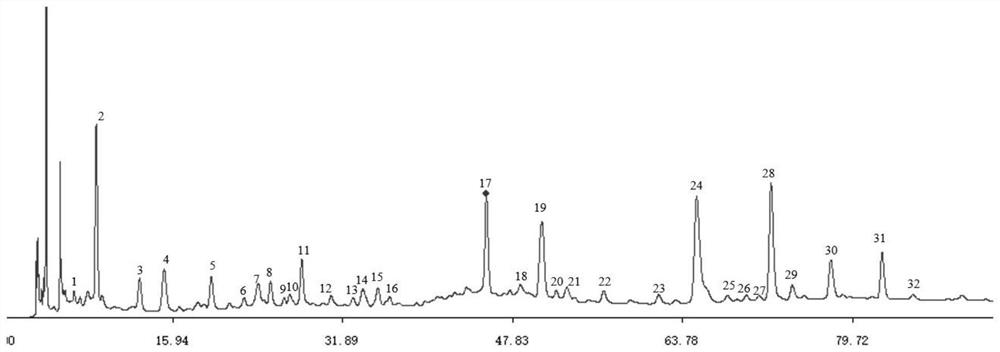
[0035] 3) Construction of fingerprint map:
[0036] Draw the test solution A and the mixed reference solution and inject it into a liquid chromatograph for high performance liquid chromatography analysis, that is, get the HPLC fingerprint of Yajunniuhazhanbo formula; establish the concentration of each substance in the mixed reference solution Or the linear relationship between the content and the peak a...
Embodiment 1
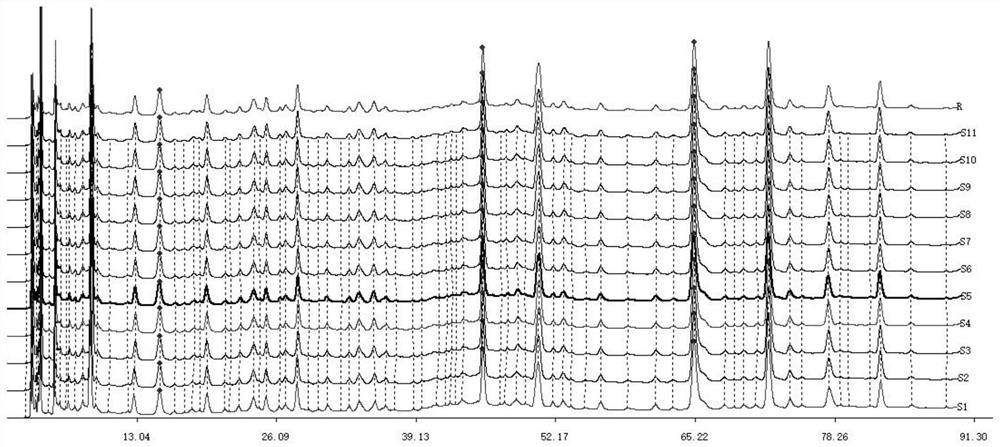
[0048] Example 1 Preparation of HPLC fingerprint of Yajunniuhazhanbo granules
[0049] 1. Experimental materials
[0050] Instruments: LC-20A high performance liquid chromatograph (DAD detector, Shimadzu); KQ-300B ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.); XS105DU analytical balance (Switzerland METTLER TOLEDO).
[0051] Reagents: methanol (batch number: 10044118, Sinopharm Chemical Reagent Co., Ltd.), acetonitrile (batch number: 193502, Thermo Fisher Scientific (China) Co., Ltd.), glacial acetic acid (batch number: 20190530, Tianjin Guangfu Technology Development Co., Ltd.) , pure water (batch number: 3212TJ, Wahaha Pure Water Co., Ltd.).
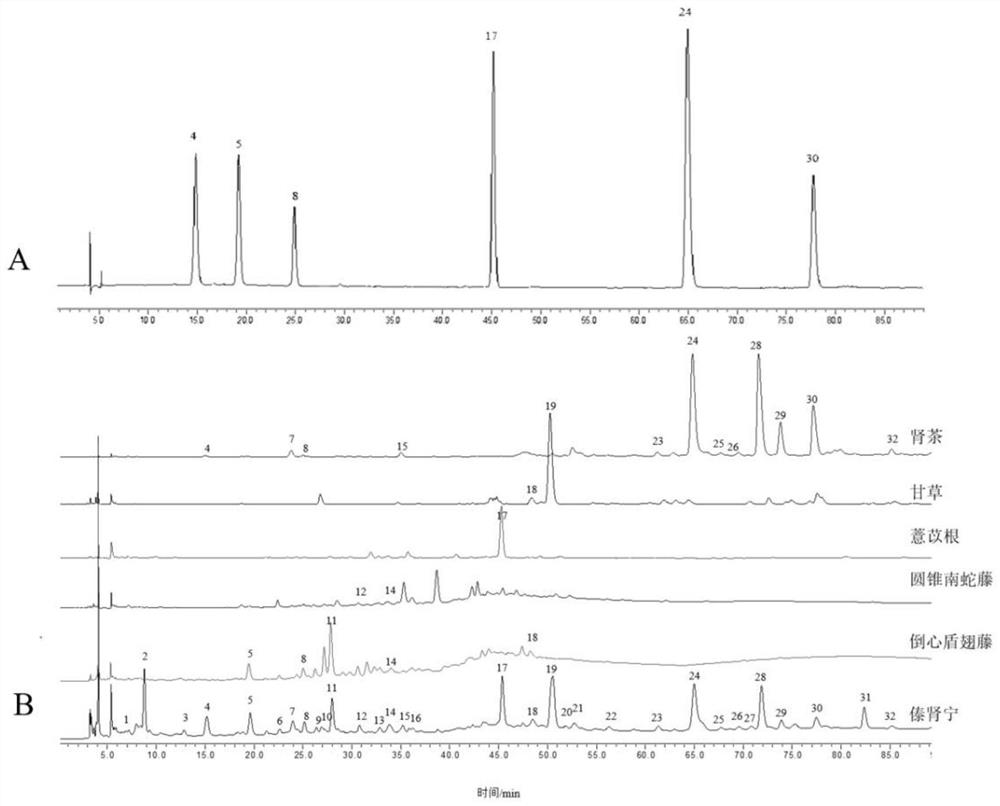
[0052] Samples to be tested: 11 batches of Yajun Niuha Zhanbo Granules (S1-S11, batch numbers are 200514, 200610, 200612, 200613, 200614, 200710, 200711, 200712, 200713, 200714, 200810, 10g / bag) and dumplings Shield-winged vine Aspidopterys obcordata cane), kidney tea (Lamiaceae plant kidney tea Clerodendranthus sp...
Embodiment 2
[0081] Example 2 Determination of the content of six active ingredients in Yajunniuhazhanbo granules
[0082] 1. Methodological investigation
[0083] 1. Examination of linear relationship
[0084] Prepare mixed reference solution according to item "2.2", inject 2 μL, 4 μL, 8 μL, 10 μL, 12 μL, 16 μL, 20 μL respectively, take the peak area as the ordinate and the injection volume of the reference substance as the horizontal coordinates, perform linear regression, draw standard curves, and fit linear equations, calculate linear range and R 2 value. The results of the linear relationship investigation are shown in Table 1. It can be seen that danshensu, protocatechuic acid, protocatechuic aldehyde, p-coumaric acid, rosmarinic acid, and salvianolic acid B are in the range of 8.0-80 μg·mL, respectively. -1 , 3.2~32 μg·mL -1 , 0.6~6.0 μg·mL -1 , 1.8~18 μg·mL -1 , 7.4~74 μg·mL -1 , 5.6~56 μg·mL -1 There is a good linear relationship with the peak area in the range, R 2 All ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com