Primer group for detecting nucleic acid of central nervous infection pathogen, product and application
A central nervous system and pathogen technology, applied in the biological field, can solve the problem of incomplete pathogens, achieve high sensitivity, high accuracy, and shorten the detection cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 The application reaction system is established
[0079] By culturing and analyzing the central nervous system infection samples, the pathogen types of such infected samples are determined. HSV-1), herpes simplex virus 2 (Herpes simplex virus 2, HSV-2), varicella zoster virus (VZV), cytomegalovirus (Cytomegalovirus, CMV), human herpes virus type 6 (Human herpesvirus 6, HHV-6), Epstein-Barr virus (EBV), mumps virus (Mumps virus, MuV), human parechovirus (Human parechovirus, HPeV)), bacteria (Escherichia coli, E. coli), Listeria monocytogenes (Listeria monocytogenes, LM), Neisseria meningitidis (Neisseria meningitidis, NM), Streptococcus agalactiae (Streptococcusagalactiae, GBS), Streptococcus pneumoniae (Streptococcus pneumoniae, SP), Haemophilus influenzae (Hi), Mycobacterium tuberculosis (MTB)), fungi (Cryptococcus neoformans (CN)), mycoplasma pneumonia (MP).
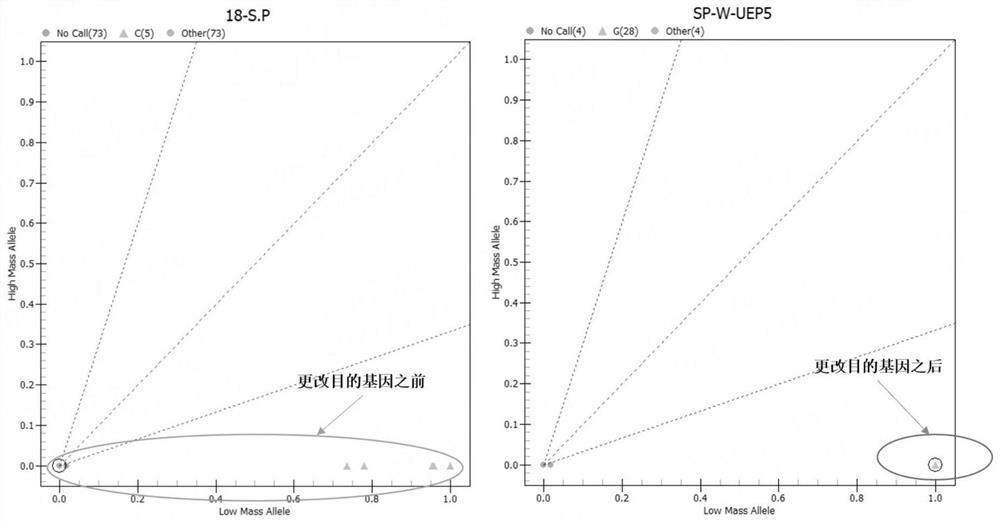
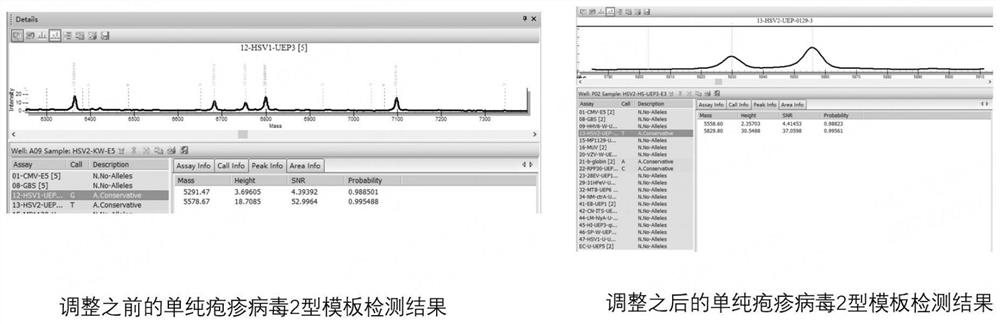
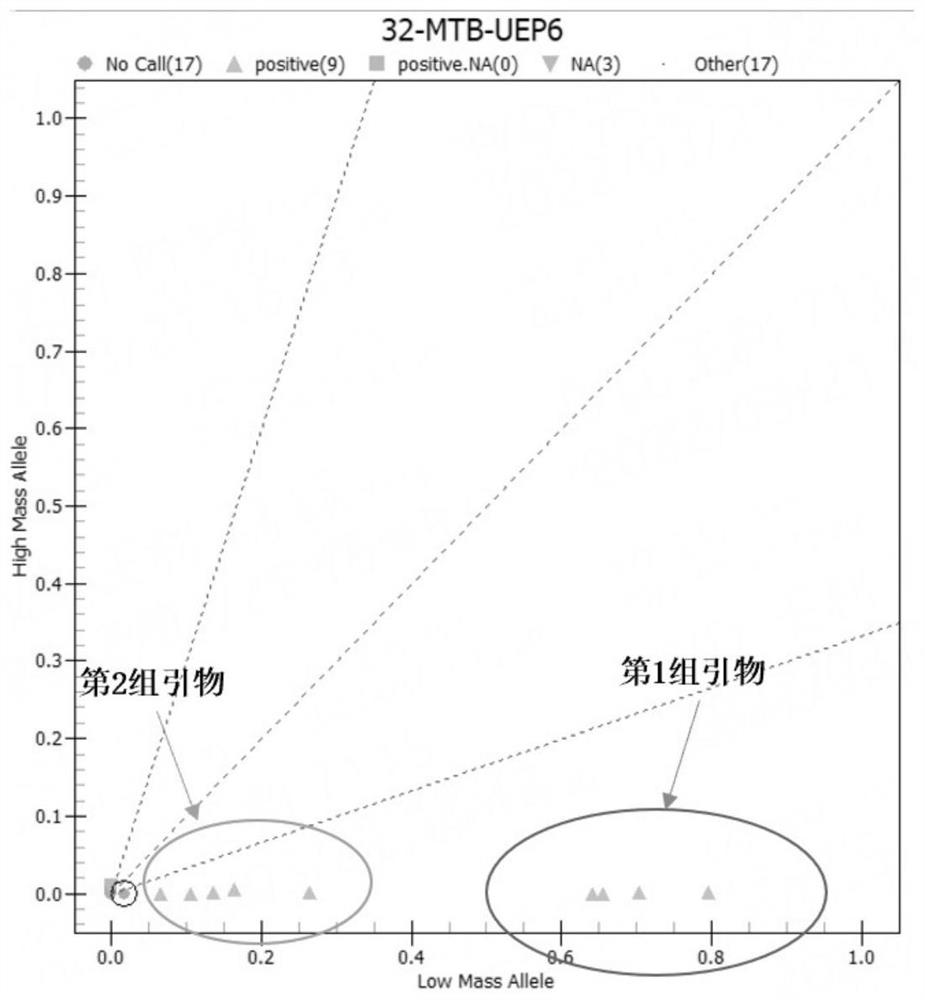
[0080] Further, in this example, by exploring the specific genes of each detection pathogenic i...
Embodiment 2
[0128] Example 2 Confirmation of the minimum detection limit of the application
[0129] After confirming the optimal reaction system in this example, each detection index is used for the minimum detection limit confirmation test using the plasmid synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. designed according to the specific detection gene. The verification scheme is as follows:
[0130]The plasmids with known concentrations of each indicator were diluted to three gradients of 50copies / μL, 10copies / μL, and 5copies / μL, and each gradient was detected twice; the lowest concentration that could be detected 100% was taken as the lowest detection limit of each indicator. , and then at this concentration, repeat the detection 5 times, and all 5 times are detected, then the concentration can be determined as the minimum detection limit of the indicator. The specific verification process is as follows: first, the amplification primer MIX and the extension primer MIX are ...
Embodiment 3
[0133] Example 3 Clinical sample detection
[0134] In the process of system optimization in this embodiment, each detection site has at least one clinical sample for detection and verification to confirm that the detection results in this embodiment are accurate. In this example, after confirming the optimal reaction system, a series of verification experiments, including accuracy and precision experiments, were carried out. The specific verification scheme is as follows:
[0135] (1) Accuracy experimental verification scheme: 18 test indicators were selected from a clinical sample for verification, and the mass spectrometry test results were more than 95% consistent with the theoretical results, and the verification passed.
[0136] (2) Validation scheme of precision experiment: Pick 5 clinical samples, and test each sample 3 times, and test 2 batches in total. If the consistency of precision between batches is greater than 95%, the verification is passed.
[0137] The spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com