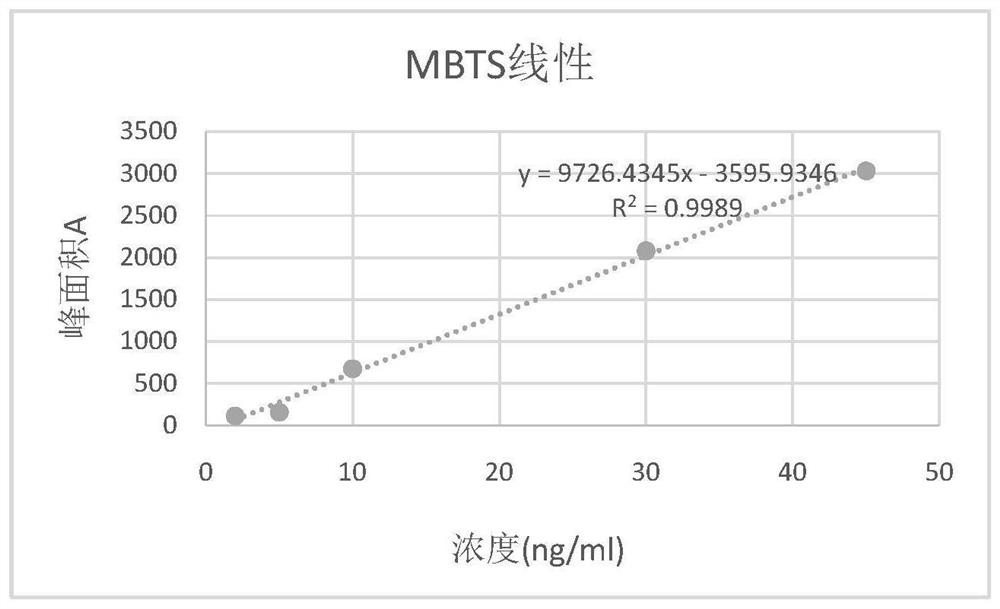
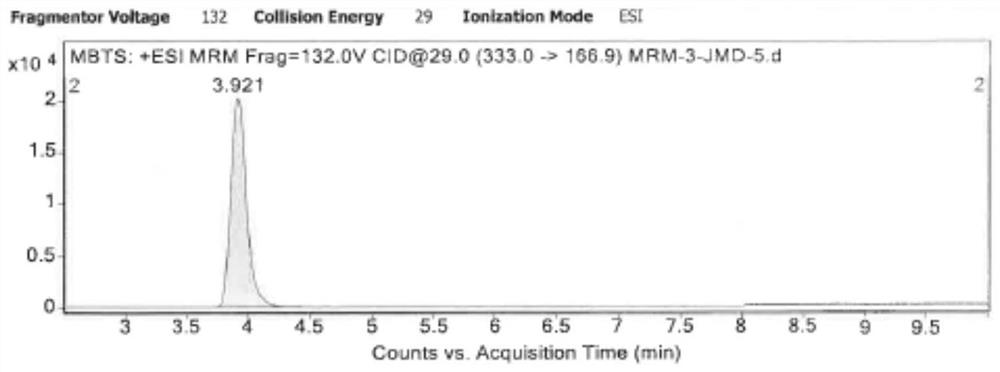
Method for detecting dibenzothiazyl disulfide in cephalosporin drugs
A technology of dibenzothiazole disulfide and a detection method is applied in the directions of measuring devices, instruments, scientific instruments, etc., and can solve the problems of inability to effectively detect residual dibenzothiazole disulfide in cephalosporins, and achieves high accuracy, Low detection limit and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The detection operation process is as follows:
[0036] 1. Instruments and Reagents
[0037] Agilent 6470 LC / MS
[0038] Standard material: dibenzothiazole disulfide
[0039] Water: Watsons
[0040] Methanol: Chromatographically pure
[0041] Dioxane: Chromatographically pure
[0042] Phosphoric acid: analytical grade
[0043] Ammonia: 29.6%
[0044] 2. Chromatographic conditions:
[0045] Column: Agilent ZORBAX Eclipse 120EC-C18 3.0×50mm, 2.7μm
[0046] Mobile phase A: 0.5% formic acid in water
[0047] Mobile phase B: methanol
[0048] Flow rate: 0.4ml / min
[0049] Column temperature: 40℃
[0050] Diluent: Dioxane-phosphoric acid (85:15)
[0051] Elution gradient:
[0052] time (min) A(%) B(%) 0 25 75 8 10 90 8.01 25 75 11 25 75
[0053] 3. Mass Spectrometry Conditions
[0054] Scan Mode: MRM(+)
[0055] Interface: AJS-ESI
[0056] Drying gas flow rate: 5.0L / min
[0057] Drying gas temperature: 300℃
[0058] Sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com