Catalyst and preparation method of 2, 7-octadiene-1-ol
A catalyst and octadiene technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of hydroxyl compounds, can solve the problems of low activity, long reaction time, and large loss of active metals, and achieve high catalytic activity and high Reactivity, activity-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] In the present invention, the preparation method of the catalyst preferably includes: in a protective atmosphere, the catalyst is obtained after the organic phosphorus ligand, the palladium salt and the solvent are mixed and reacted.
[0054]In the present invention, the catalyst is preferably a catalyst for temperature-controlled solid-liquid separation.
[0055] In the present invention, the catalyst preferably dissolves in a solvent system at a temperature above 70°C, and precipitates into a solid in the solvent system at 20°C to achieve solid-liquid separation.
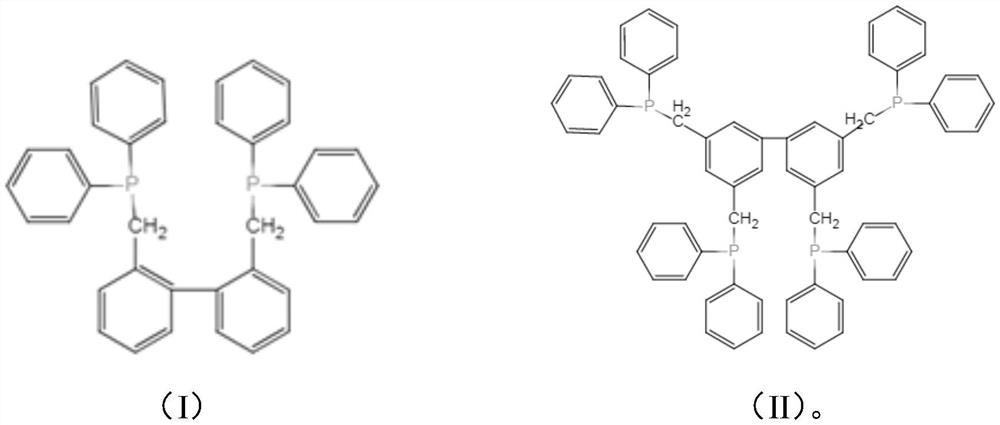
[0056] The organophosphine ligands prepared by the present invention are characterized.
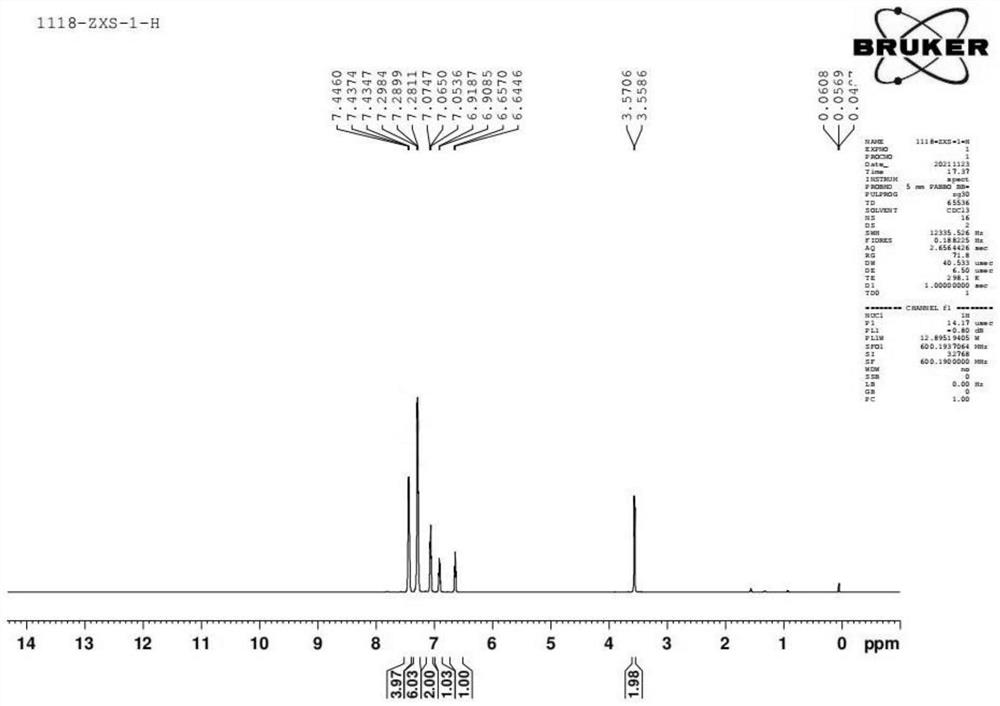
[0057] see figure 1 , figure 1 The nuclear magnetic H spectrum of the organophosphine ligand with the structure represented by formula (I) prepared in the present invention.
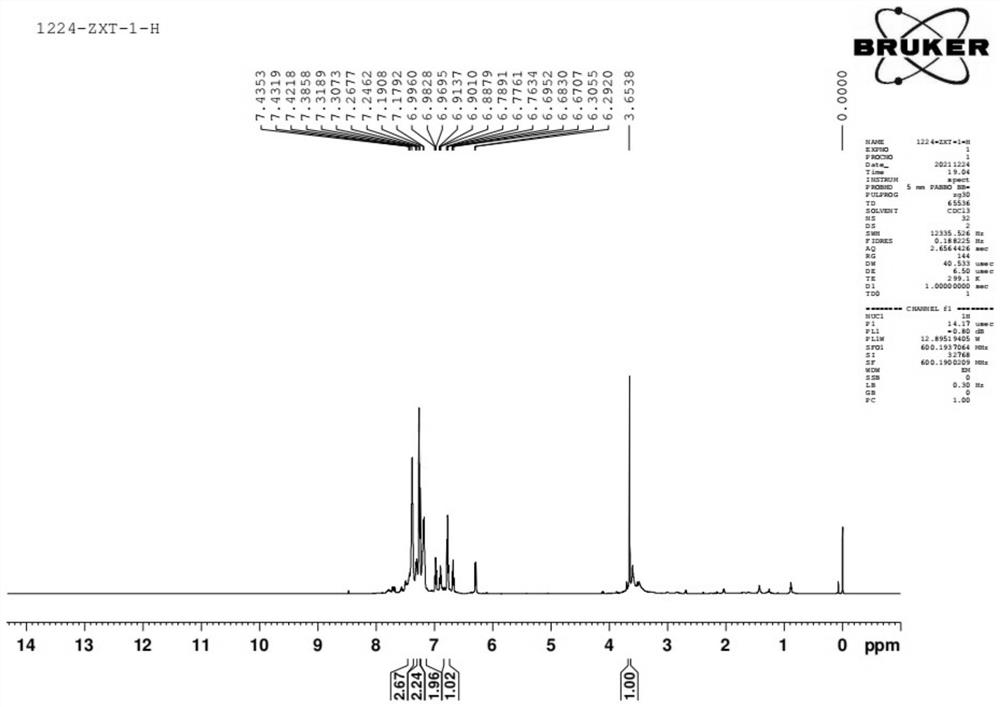
[0058] see figure 2 , figure 2 The nuclear magnetic H spectrum of the organophosphine ligand with the structure represented by formula (I...
Embodiment 1
[0097] Weigh 0.1 g of palladium acetate, 1.0 g of ligand A, and 50 g of sulfolane into a 250-ml reaction kettle, pass nitrogen gas, and react at 20° C. for 1 h. Subsequently, 30 g of sulfolane, 80 g of water, 30 g of butadiene, and 24 g of triethylamine were added, and carbon dioxide was introduced into the reactor to reach a pressure of 0.5 Mpa, and the reaction was carried out at 70° C. for 1 h. Wherein, ligand A is first prepared by radical reaction of 2.2-dimethylbiphenyl and N-bromosuccinimide to obtain 2.2-dibromomethylbiphenyl, and then reacted with diphenylphosphine under the catalysis of n-butyllithium Prepared by reaction at -70°C. Ligand B was prepared in the same manner as Ligand A by 3.3.5.5-Tetramethylbiphenyl.
[0098] After the reaction was completed, the temperature was lowered to room temperature, the catalyst was filtered, and the sample was analyzed by gas chromatography. The conversion rate of butadiene was 92.9%, and the selectivity of 2.7-octadien-1-ol ...
Embodiment 2
[0100] The preparation and evaluation of the catalyst were carried out according to the method of Example 1. The difference was that Ligand A was replaced with Ligand B. After the reaction was completed, the temperature was lowered to room temperature, the catalyst was filtered, and the sample was analyzed by gas chromatography. The conversion rate of butadiene was 98.9%. 2.7-Octadien-1-ol selectivity 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com