Novel polypeptide and application thereof in preparation of medicine for treating skin wound or mucosal lesion
A technology for skin wounds and new peptides, applied in skin care preparations, drug combinations, skin diseases, etc., can solve problems such as difficult treatment, lingering disease, slow onset, etc., and achieve the effect of promoting repair, small molecular weight, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Screening of new polypeptide molecules
[0040] (1) The new peptide is from Sichuan Good Doctor Panxi Pharmaceutical Co., Ltd.
[0041] (2) EGFR protein target affinity screening
[0042] EGFR affinity screening: After dissolving the freeze-dried polypeptides of the present invention into powders with protein buffers, a polypeptide compound sample solution was prepared, and an appropriate concentration of EGFR solution was incubated with the polypeptide compound sample solution at room temperature for 50 minutes, and then affinity screening was performed. The centrifugation speed was 90000rpm and the time was 70min. After centrifugation, the samples were taken out and divided into upper, middle and lower layers. Appropriate volumes of acetonitrile and water were added to each layer to precipitate proteins, and the supernatant was centrifuged to collect the supernatant for mass spectrometry analysis.
[0043] EGF polypeptide was used as a positive compound con...
Embodiment 2
[0048] Embodiment 2 The chemical synthesis method of new polypeptide
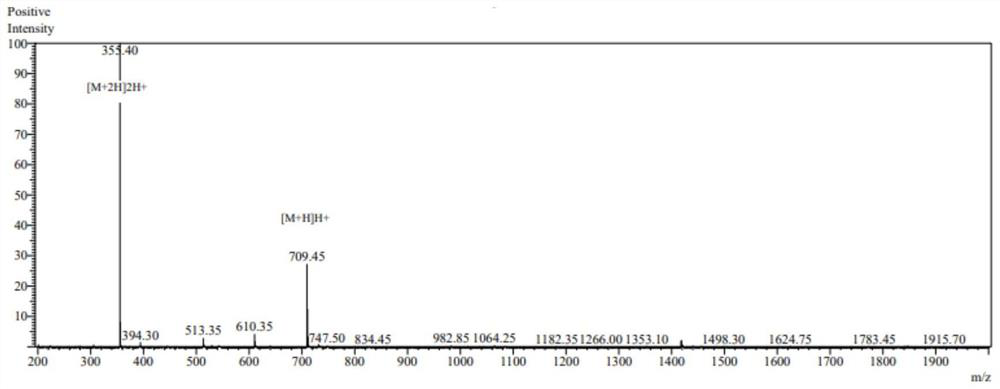
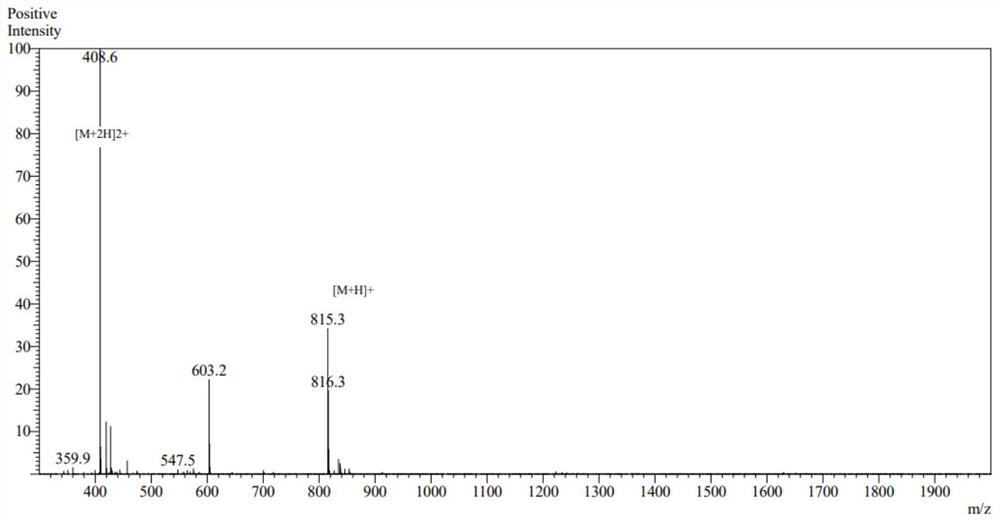
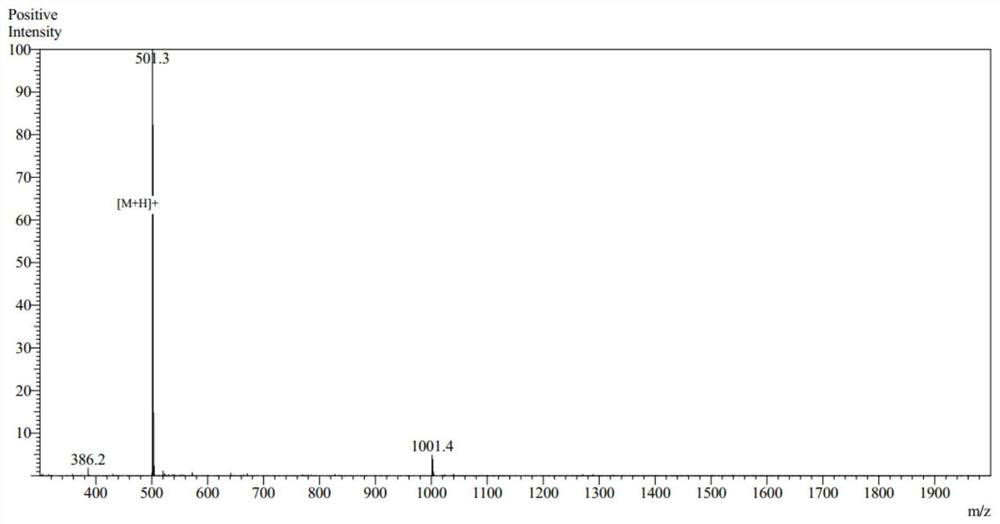
[0049] Using the conventional solid-phase synthesis method of an automatic peptide synthesizer, after resin swelling, deprotection, washing, amino acid dissolution, amino acid activation, and condensation, the chemical synthesis of new peptides was carried out, and 10 peptides were obtained. After mass spectrometry analysis, the structure was confirmed. Figures 1 to 10 is the mass spectrum of the new peptide.
Embodiment 3
[0050] Example 3 The effect of the new polypeptide PA2 on the proliferation of Hacat cells
[0051] Adjust the concentration of human immortalized keratinocytes (HaCaT cells) to 1.0 × 10 5 ~5.0×10 5 / mL for subculture at 37°C, 5% CO 2 Incubate for 24-36 hours under conditions for biological activity detection. Digest the cells with 0.25% trypsin for 5 minutes, add 1640 whole blood medium with more than 1 times the volume of trypsin to terminate the digestion, collect the cell suspension, centrifuge at 1000 RPM for 3 minutes, discard the supernatant, and add 2 mL of 1640 whole blood medium to resuspend the cells , take 20uL of cell suspension, stain with AOPI, and then use a cell counter to detect the concentration of cells in the suspension, and use 10% serum concentration of 1640 medium to make a concentration of 5 × 10 4 Inoculated in 96-well cell culture plate, 100 μL per well, i.e. 5000 cells / well, at 37°C, 5% CO 2 Incubate overnight under conditions. After 24 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com