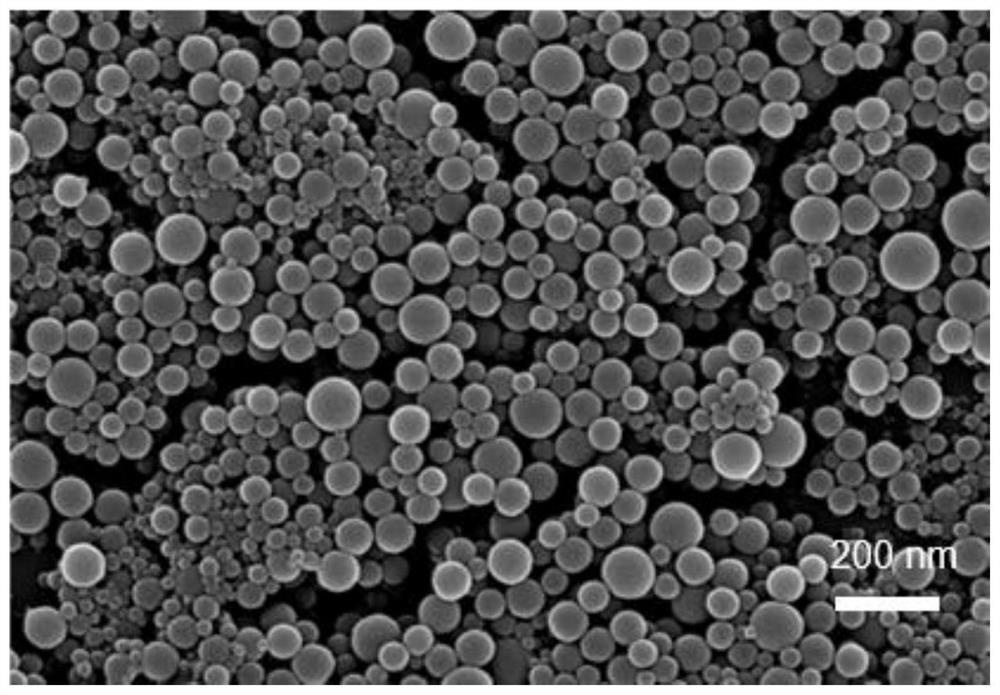
Amorphous MOF nano photosensitizer as well as preparation method and application thereof
A photosensitizer, amorphous technology, applied in the field of photosensitive materials, can solve problems such as limited penetration depth and irradiation area, difficult to obtain therapeutic effect, and reduced ROS generation, achieve good long-term stability, improve bioavailability, and improve Effect of PDT effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
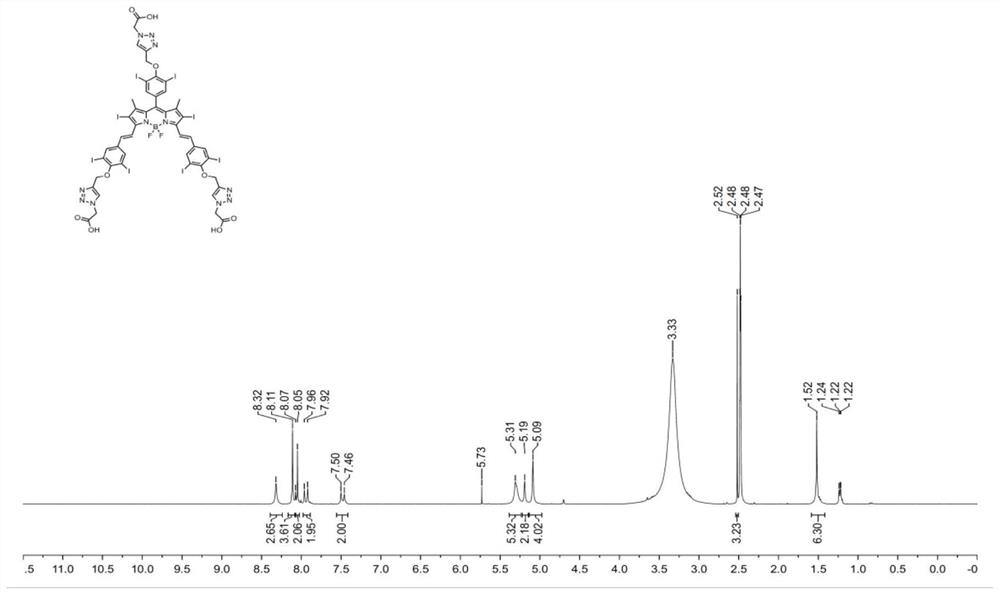
[0059] Embodiment 1 Near-infrared photosensitizer (BDP-I 8 ) preparation
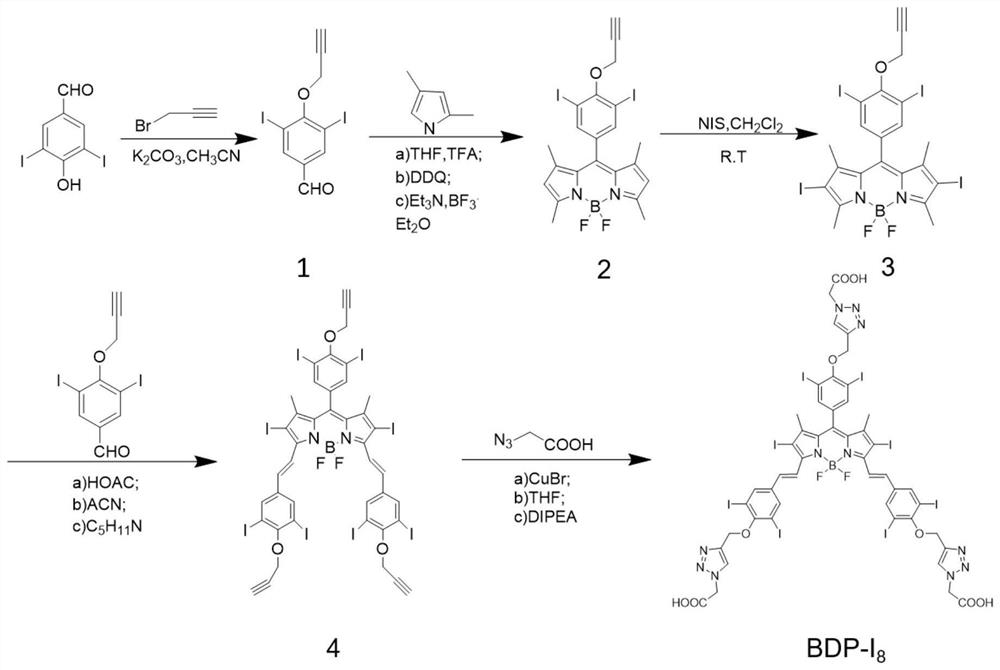
[0060] The present embodiment relates to a near-infrared photosensitizer BDP-I 8 The synthesis of , its synthetic route is as figure 1 shown, including the following steps:
[0061] (1) Synthesis of compound 1: 3,5-diiodo-p-hydroxybenzaldehyde and bromopropyne were added to the reaction vessel in a molar ratio of 1:3, then potassium carbonate in a molar ratio of 1:1 with bromopropyne was added, and then added Acetonitrile 50mL was stirred at 80°C for 12h. After the reaction, extract with dichloromethane and water, collect the organic layer, and concentrate on a rotary evaporator to obtain a crude product. The crude product is separated by silica gel column chromatography (eluent is petroleum ether-dichloromethane system) to obtain Compound 1, 85% yield.
[0062] (2) Synthesis of compound 2: Compound 1 and 2,4-dimethylpyrrole were added to the reaction vessel in a molar ratio of 1:2, and then tetrah...
Embodiment 2
[0066] Example 2 BDP-I 8 preparation
[0067] The present embodiment relates to a near-infrared photosensitizer BDP-I 8 The synthesis of , its synthetic route is as figure 1 shown, including the following steps:
[0068] (1) Synthesis of compound 1: 3,5-diiodo-p-hydroxybenzaldehyde and bromopropyne were added to the reaction vessel in a molar ratio of 1:5, then potassium carbonate in a molar ratio of 1:1 with bromopropyne was added, and then added Acetonitrile 80 mL was stirred at 60 °C for 12 h. After the reaction, extract with dichloromethane and water, collect the organic layer, and concentrate on a rotary evaporator to obtain a crude product. The crude product is separated by silica gel column chromatography (eluent is petroleum ether-dichloromethane system) to obtain Compound 1, 70% yield.
[0069] (2) Synthesis of compound 2: Compound 1 and 2,4-dimethylpyrrole were added to the reaction vessel in a molar ratio of 1:3, and then tetrahydrofuran 100 times the weight of...
Embodiment 3
[0073] Example 3 BDP-I 8 preparation
[0074] The present embodiment relates to a near-infrared photosensitizer BDP-I 8 The synthesis of , its synthetic route is as figure 1 shown, including the following steps:
[0075] (1) Synthesis of compound 1: 3,5-diiodo-p-hydroxybenzaldehyde and bromopropyne were added to the reaction vessel in a molar ratio of 1:6, then potassium carbonate in a molar ratio of 1:2 with bromopropyne was added, and then added Acetonitrile 50mL was stirred at 80°C for 12h. After the reaction, extract with dichloromethane and water, collect the organic layer, and concentrate on a rotary evaporator to obtain a crude product. The crude product is separated by silica gel column chromatography (eluent is petroleum ether-dichloromethane system) to obtain Compound 1, 80% yield.
[0076](2) Synthesis of compound 2: Compound 1 and 2,4-dimethylpyrrole were added to the reaction vessel in a molar ratio of 1:4, and then tetrahydrofuran 100 times the weight of 2,4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com