Preparation method of bis (3-amino-4-hydroxyphenyl) hexafluoropropane
A technology of hydroxyphenyl and hexafluoropropane is applied in the field of preparation of bis-hexafluoropropane, which can solve the problems of low actual product yield and severe production process, and avoid hydrogenation reduction reaction, waste acid avoidance, and nitration reaction avoidance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
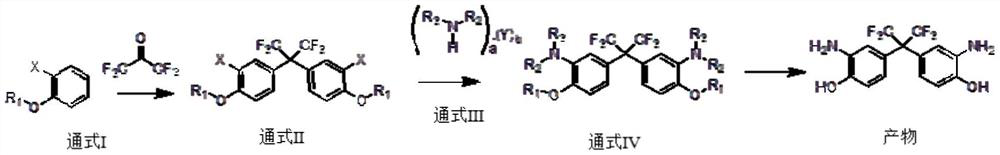
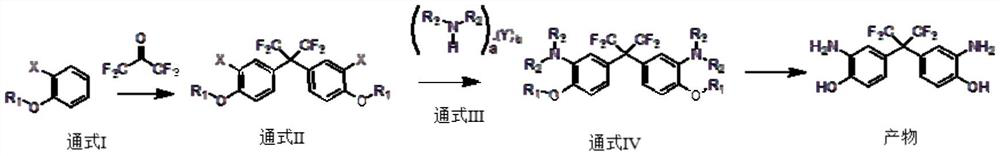
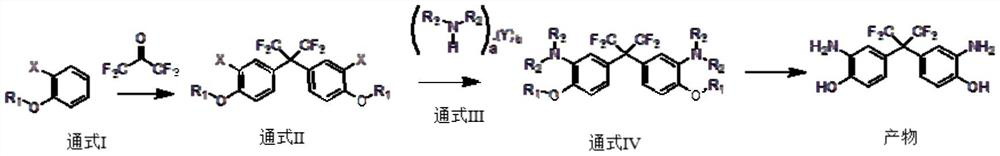
[0039] A first aspect of the present invention provides a method for preparing bis(3-amino-4-hydroxyphenyl) hexafluoropropane, the specific steps comprising:
[0040] S1: in the presence of catalyst A, general formula I reacts with hexafluoroacetone to obtain general formula II;
[0041] S2: in the presence of catalyst B, general formula II reacts with general formula III to obtain product;
[0042] The general formula I is the ortho-substituent of phenol and its derivatives;
[0043] In the general formula II, the group R1 is one of H, a C1-C8-containing hydrocarbon group or a heteroatom-substituted hydrocarbon group;
[0044] The general formula III is an amine compound, and the amine compound is an amine monomer and / or an amine salt;
[0045] In the general formula III, a is a natural number ≥ 1, b is a non-negative integer ≥ 0, and Y is H 2 O, one of organic acids, inorganic acids;
[0046] R2 and R3 can be the same or different;
[0047] When R1, R2 and R3 are all H,...
Embodiment 1
[0095] Embodiment 1 provides a preparation method of bis(3-fluoro-4-hydroxyphenyl) hexafluoropropane: in the presence of catalyst A, general formula I reacts with hexafluoroacetone to obtain general formula II; the synthesis reaction formula is as follows :
[0096]
[0097] The preparation steps are as follows: 50g of solvent I, 16.82g of general formula I, and 2.58g of catalyst A are sequentially added to the glass reaction vessel, the reaction temperature is set to 30°C, and 14.94g of hexafluoroacetone gas is slowly introduced to react, and the gas is completely passed through. After adding, the temperature was raised to 40°C and reacted for 12 hours, then cooled to 25°C to obtain a reaction solution, washed with saturated aqueous sodium bicarbonate solution until the pH was 7-8, collected the organic phase, washed the organic phase with pure water, and concentrated the organic phase to obtain The crude product was recrystallized with 57 g of ethanol, and the recrystalli...
Embodiment 2
[0101] Embodiment 2 provides a preparation method of bis(3-chloro-4-hydroxyphenyl) hexafluoropropane: in the presence of catalyst A, general formula I reacts with hexafluoroacetone to obtain general formula II; the synthesis reaction formula is as follows :
[0102]
[0103] The preparation steps are as follows: 50g of solvent I, 19.29g of general formula I, and 2.73g of catalyst A are sequentially added to the glass reaction vessel, the reaction temperature is set to 28°C, and 14.94g of hexafluoroacetone gas is slowly introduced to react, and the gas is completely passed through. After adding, the temperature was raised to 85°C and reacted for 15 hours, and then the temperature was lowered to 25°C to obtain a reaction solution. The reaction solution was washed with a saturated aqueous sodium bicarbonate solution until the pH was 7 to 8, and 50 g of dichloromethane was added to extract and separate the layers. The organic phase was washed with water, the organic phase was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com