Polyester depolymerization or cyclic ester synthesis catalyst as well as preparation method and application thereof
A catalyst and polyester technology, which is applied in the direction of carboxylate preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc. It can solve the problems of large catalyst consumption, slow reaction rate, and low utilization value, etc. problems, avoiding equipment and process design challenges, facilitating industrial production, and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
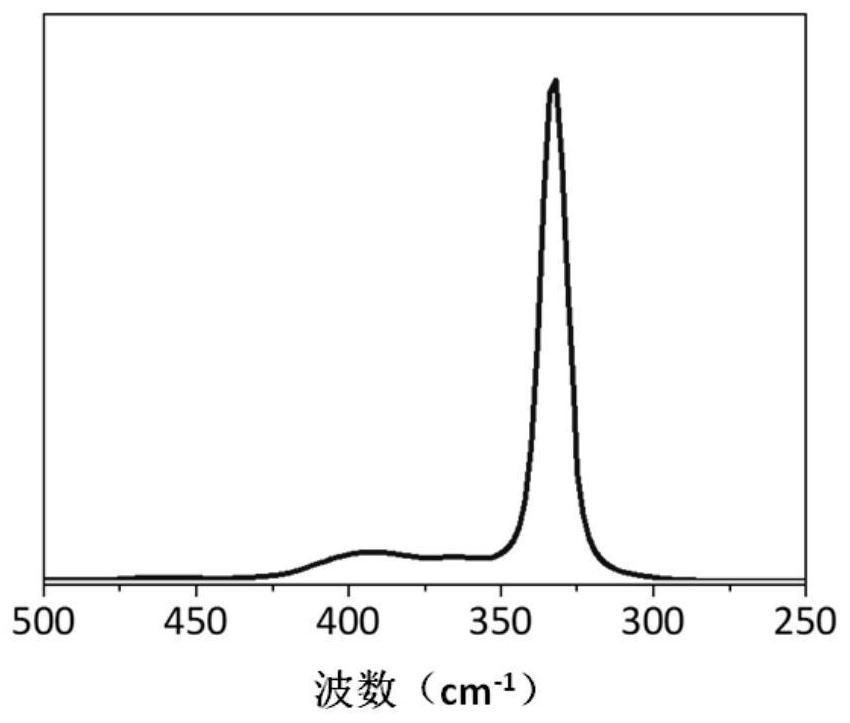
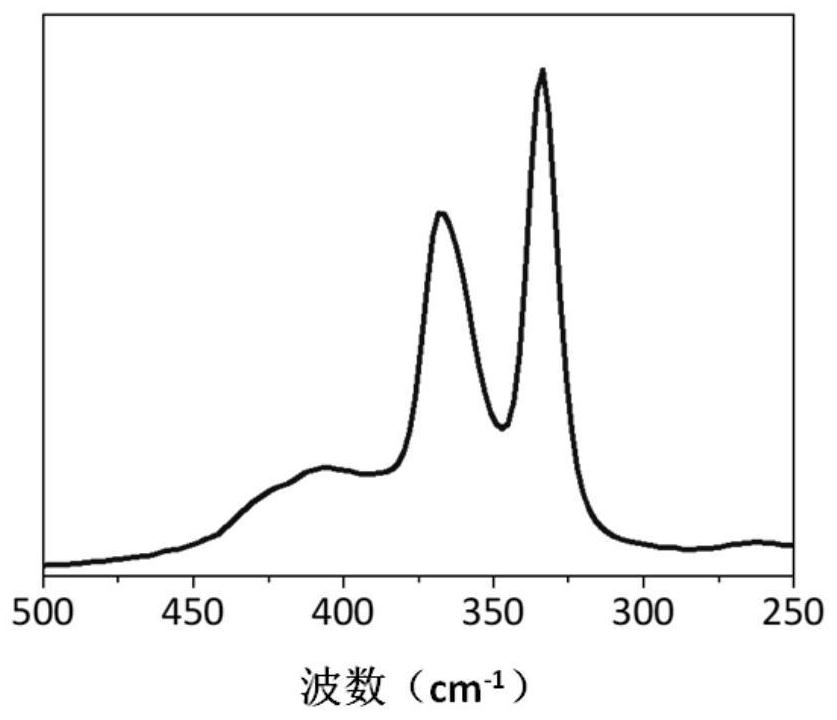
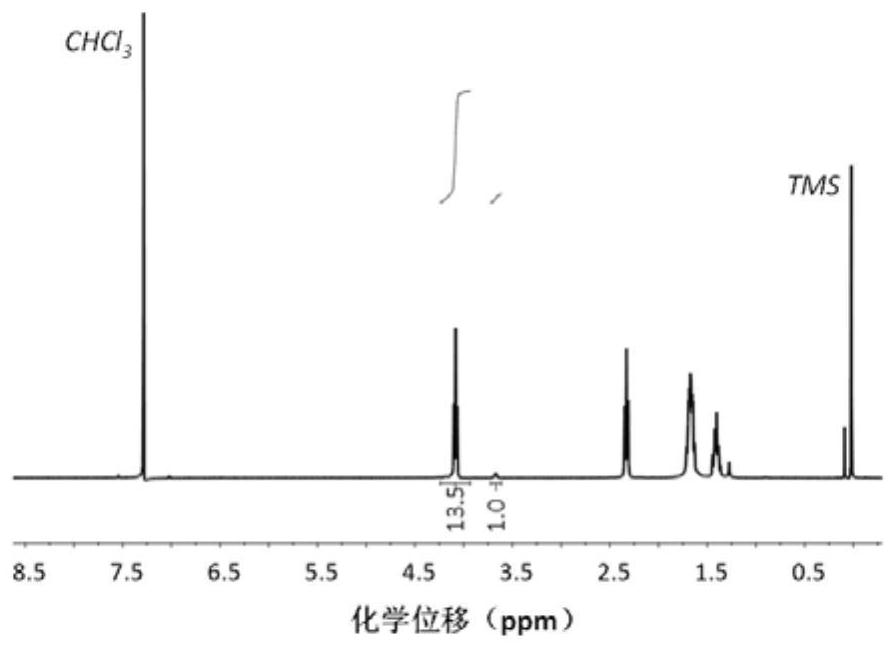
Image
Examples
Embodiment 1
[0075] (1) 40mmol of 1-methylimidazole and 40mmol of 8-chloro-n-octanol were added to the round-bottomed flask, and the reaction was stirred for 48h under heating in an oil bath at 100°C in a nitrogen atmosphere, and then washed with ethyl acetate and dried to obtain 1 -Hydroxyoctyl-3-methylimidazolium chloride (Cl –+ MIm-C 8 -OH). (2) 20mmol Cl –+ MIm-C 8 -OH with 20mmol FeCl3 Add to a round-bottomed flask, stir and react in an oil bath at 80 °C in a nitrogen atmosphere for 0.5 h to obtain FeCl 3 -Cl –+ MIm-C 8 -OH. (3) 10mmol FeCl 3 -Cl –+ MIm-C 8 -OH and 100mmolδ-VL were added to a round-bottomed flask, and the reaction was stirred for 4h under heating in an oil bath at 140°C in a nitrogen atmosphere, and FeCl was prepared by ring-opening polymerization. 3 -Cl –+ MIm-C 8 -poly(δ-VL) 10 -OH. The long-chain structure of the products of step (1) and step (2) is a long alkyl chain, and the number of carbon atoms in the main chain=8; the long-chain structure of the...
Embodiment 2
[0077] (1) 40mmol of 1-methylimidazole and 48mmol of 3-chloro-1-propanol were added to the round-bottomed flask, and the reaction was stirred for 48h under heating in an oil bath at 80°C in a nitrogen atmosphere, washed with ethyl acetate and dried. Obtain 1-hydroxypropyl-3-methylimidazolium chloride (Cl –+ MIm-C 3 -OH). (2) 20mmol Cl –+ MIm-C 3 -OH with 20mmol FeCl 3 Add it to a round-bottomed flask, stir and react for 4h under heating in an oil bath at 25°C in a nitrogen atmosphere to obtain FeCl 3 -Cl –+ MIm-C 3 -OH. (3) 10mmol FeCl 3 -Cl –+ MIm-C 3 -OH and 50mmolδ-VL were added to the round-bottomed flask, and the reaction was stirred for 8h under the heating of an oil bath at 120°C in a nitrogen atmosphere, and FeCl was obtained by ring-opening polymerization. 3 -Cl –+ MIm-C 3 -poly(δ-VL) 5 -OH. The long-chain structure of the product in step (3) is an aliphatic polyester chain, and the number of carbon atoms in the main chain=5*5+3=28.
Embodiment 3
[0079] (1) 40mmol of 1-methylimidazole and 32mmol of 2-bromo-1-ethanol were added to the round-bottomed flask, and the reaction was stirred for 24h under heating in an oil bath at 60°C in a nitrogen atmosphere, washed with ethyl acetate and dried to obtain 1-Hydroxyethyl-3-methylimidazolium bromide (Br –+ MIm-C 2 -OH). (2) 20mmol Br –+ MIm-C 2 -OH with 20mmol FeCl 3 Add it to a round-bottomed flask, and stir the reaction for 12h under the heating of an oil bath at 25°C in a nitrogen atmosphere to obtain FeCl 3 -Br –+ MIm-C 2 -OH. (3) 10mmol FeCl 3 -Br –+ MIm-C 2 -OH and 300mmolδ-VL were added to a round-bottomed flask, and the reaction was stirred for 2h under heating in an oil bath at 160°C in a nitrogen atmosphere, and FeCl was prepared by ring-opening polymerization. 3 -Br –+ MIm-C 2 -poly(δ-VL) 30 -OH. The long-chain structure of the product in step (3) is an aliphatic polyester chain, and the number of carbon atoms in the main chain=5*30+2=152.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com