Aza fused ring compound and synthesis method thereof
A synthesis method and compound technology, applied in the direction of steroids, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., can solve the problems of overall synthesis complexity, limited product range, etc., and achieve good functional group compatibility Sexual and efficient introduction of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
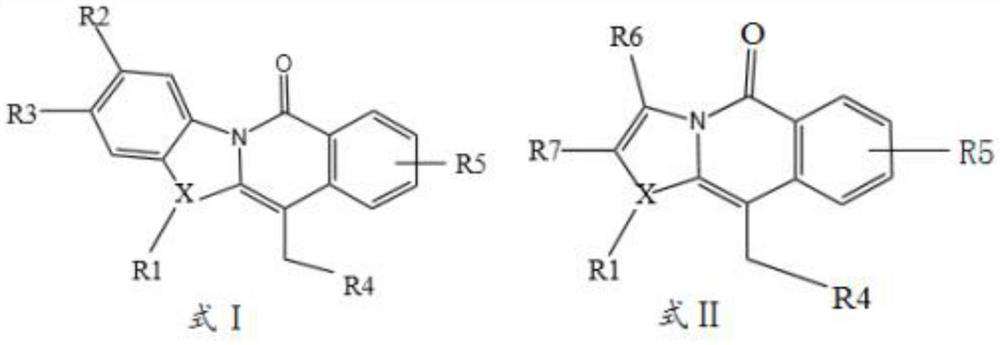
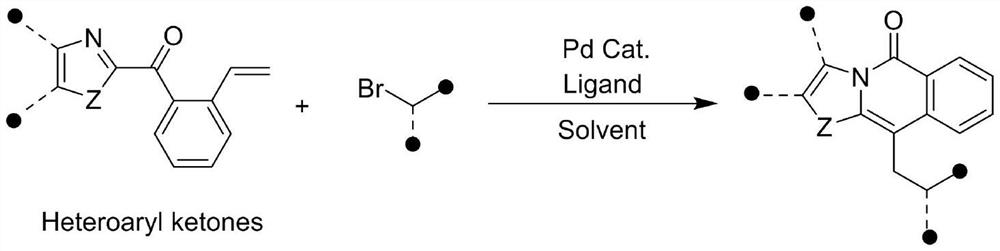
[0039] The invention provides a method for synthesizing aza-fused-ring compounds, which comprises the following steps: taking substituted or unsubstituted heteroaryl ketone and halide as raw materials, and dearomatizing in the presence of a palladium catalyst, a ligand and a solvent The reaction generates a spiro-ring intermediate, and the spiro-ring intermediate undergoes the intramolecular acyl migration driven by aromatization to induce the skeleton rearrangement of the heteroaryl ketone to obtain the compound represented by formula I or formula II:
[0040]
[0041] Wherein, X is selected from sulfur atom, nitrogen atom or oxygen atom;
[0042] R2, R3, R4, R5, R6, R7 are independently selected from alkyl, aryl, ester, sulfonyl, mercapto, amino, boryl, halogen, thioether, sulfinyl, phospholipid, alkene, alkyne group, selenide, fluorine-containing alkane or deuterated atom;
[0043] The synthetic route is as follows:
[0044]
[0045] The preparation of (benzo)imidaz...
Embodiment 1
[0068] Using (1-methyl-1H-benzo[d]imidazol-2-yl)(2-vinylphenyl)methanone 1 (0.1 mmol) as a model substrate, ethyl difluorobromoacetate (BrCF 2 COOEt) (0.15mmol, 1.5 equiv) as coupling agent, palladium dichloride (0.01mmol, 10mol%) as catalyst, 1,5-bis(diphenylphosphine)pentane (dpppent) (0.012mmol, 12mol%) ) as a ligand, and sodium carbonate (0.1 mmol, 1.0 equiv.) was added to react 24 in a mixed solvent of 1,4-dioxane and tetrahydrofuran (volume ratio 1:2) (1.0 mL) at a temperature of 130 °C. Within hours, product 1 was obtained in 90% yield.
[0069]
[0070] Yellow solid, Mp=253–254°C. 1H NMR (400MHz, CDCl 3 )δ=8.79–8.77 (m, 1H), 8.50–8.48 (m, 1H), 7.59–7.53 (m, 2H), 7.34–7.26 (m, 2H), 7.19–7.14 (m, 1H), 7.05– 7.02 (dd, J=1.2Hz, J=8.0Hz, 1H), 4.20–4.15 (m, 2H), 3.87–3.81 (m, 5H), 1.19–1.16 (m, 3H). 13C NMR (100MHz, CDCl) 3 )δ=164.36(t,J=32.0Hz), 159.59,140.87,138.07,135.08,132.04,127.57,127.50,125.70,122.43,121.36, 121.14,118.91,116.84,115.18,106.73,79.91,63.19,32.68,...
Embodiment 2
[0072] Using (1-methyl-1H-benzo[d]imidazol-2-yl)(2-vinylphenyl)methanone 1 (0.1 mmol) as a model substrate, ethyl difluorobromoacetate (BrCF 2 COOEt) (0.1 mmol, 1.0 equiv) as coupling agent, palladium acetate (0.01 mmol, 10 mol%) as catalyst, 1,5-bis(diphenylphosphine)pentane (dpppent) (0.012 mmol, 12 mol%) as catalyst ligand, and sodium carbonate (0.12 mmol, 1.2 equiv.) was added, and the mixed solvent of 1,4-dioxane and tetrahydrofuran (volume ratio 1:2) (1.0 mL) was reacted for 24 hours at a temperature of 130 °C, Product 1 was obtained in 54% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com