Enaminone boron complex fluorescent material as well as preparation method and application thereof
A technology of enaminone boron and fluorescent materials, applied in luminescent materials, chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, etc., can solve the problems of limited electron-pulling ability and luminescence limitation, and achieve high The effect of external quantum efficiency and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
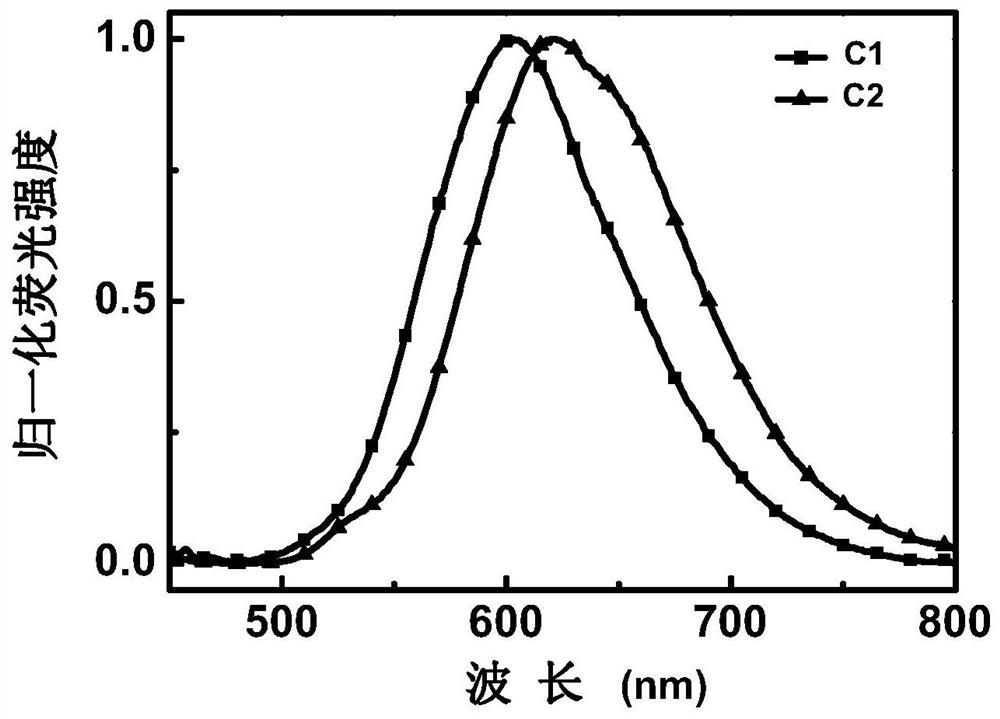
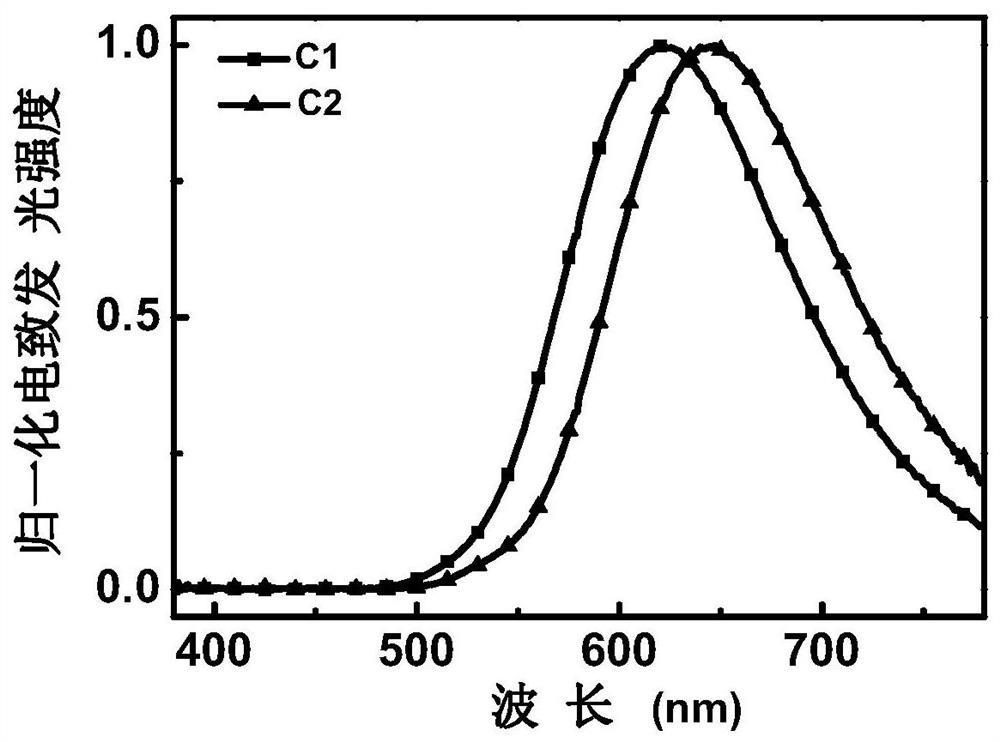
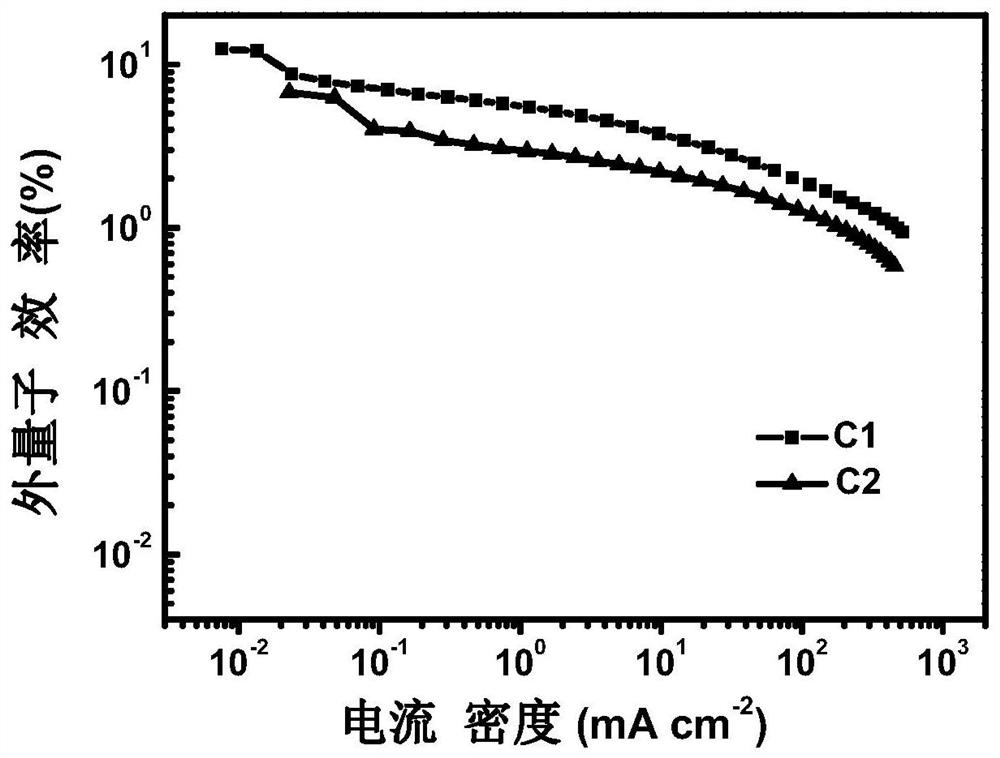
[0038] Embodiment 1: an enaminone boron complex thermally activated delayed fluorescent material with a structure of C1, the synthetic route is as follows:
[0039]
[0040] (1) Synthesis of intermediates with structure 1: in an inert atmosphere, Z-1-(4-bromophenyl)-3-(anilino)propenone (3.0 mmol, 0.90 g), 9,10-di Hydrogen-9,9-dimethylacridine (4.5mmol, 0.94g), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 0.15mmol, 137mg), tri-tert-butylphosphine tetrafluoroborate ((t-Bu) 3 PH-BF 4 , 1.2mmol, 348mg) and sodium tert-butoxide (9.0mmol, 0.86g) were added to a 250mL two-necked flask, and then the reaction system was pumped and ventilated three times. After that, 80 mL of anhydrous toluene solvent was introduced into the reaction system, and finally the reaction system was heated to 95° C. and reacted at this temperature for 12 h. The reaction was stopped after the disappearance of the raw materials was detected by TLC plate. After cooling the reaction system to ro...
Embodiment 2
[0042] Embodiment 2: a thermally activated delayed fluorescent material of an enaminone boron complex with a structure of C2, the synthetic route is as follows:
[0043]
[0044] (1) Synthesis of intermediates with structure 2: In an inert atmosphere, Z-1-(4-bromophenyl)-3-(4-cyanoanilino)propenone (3.0 mmol, 0.98 g), 9 , 10-dihydro-9,9-dimethylacridine (4.5mmol, 0.94g), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 0.15mmol, 137mg), tri-tert-butylphosphine tetrafluoroborate ((t-Bu) 3 PH-BF 4 , 1.2mmol, 348mg) and sodium tert-butoxide (9.0mmol, 0.86g) were added to a 250mL two-necked flask, and then the reaction system was pumped and ventilated three times. After that, 80 mL of anhydrous toluene solvent was introduced into the reaction system, and finally the reaction system was heated to 95° C. and reacted at this temperature for 12 h. The reaction was stopped after the disappearance of the raw materials was detected by TLC plate. After cooling the reaction sys...
Embodiment 3
[0046] Embodiment 3: a kind of structure is C3 enamino ketone boron complex fluorescent material, the synthetic route is as follows:
[0047] Intermediate 2 was synthesized according to step (1) in Example 2.
[0048] Synthesis of C3: The raw material intermediate 2 (1 mmol, 0.46 g) and tris(4-fluorophenyl) boron (1.5 mmol, 0.44 g) were added to a 250 mL round-bottomed flask, and argon was replaced under vacuum, and then anhydrous toluene was introduced 60 mL, the reaction body was reacted at 110 °C overnight. After the reaction, the solvent was spin-dried, and 0.47 g of solid was obtained by silica gel column separation, and the yield was 70%. MALDI-TOF MS: Calculated: 655.3 [M] + , measured value: 655.2[M] + .
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com