Preparation and application of polypeptide for promoting bone regeneration or bone formation
A bone regeneration and multipurpose technology, applied in the field of biomedicine, can solve the problems of increasing the risk of cancer development, poor protein stability, and hindering clinical application, etc., and achieve the effect of simple structure, small molecular weight, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The present embodiment provides a method for preparing a polypeptide that promotes the osteogenic function of BMP2, comprising the following steps:
[0061] 1. Construction of Overexpression Plasmid
[0062] 1.1 Obtaining primers
[0063] Using pGEX-4T-1 as a vector, BamH I and Not I were selected as restriction sites, and primers for different LRR fragments in OMD were designed: OMD_LRRNT, OMD_LRR1-3, OMD_LRR4-6, OMD_LRR7-9, OMD_LRR10-11.
[0064] The amino acid sequence (SEQ ID NO:2) of OMD_LRRNT is: VPFHQYTL GCVSECFCPT NFPSSMYCDNRKLKTIPNIP M;
[0065] The amino acid sequence (SEQ ID NO:3) of OMD_LRR1-3 is: HIQQLYLQF NEIEAVTANS FINATHLKEINLSHNKIKSQ KIDYGVFAKL PNLLQLHLEH NNLEEFPFPL PKSL;
[0066] The amino acid sequence (SEQ ID NO: 4) of OMD_LRR4-6 is: ERLLLG YNEISKLQTN AMDGLVNLTMLDLCYNYLHD SLLKDKIFAK MEKLMQLNLC SNRLESMPPG LPS;
[0067] The amino acid sequence (SEQ ID NO: 5) of OMD_LRR7-9 is: SLMYLSL ENNSISSIPE KYFDKLPKLHTLRMSHNKLQ DIPYNIFNLP NIVELSVGHN KLKQ;
[00...
Embodiment 2
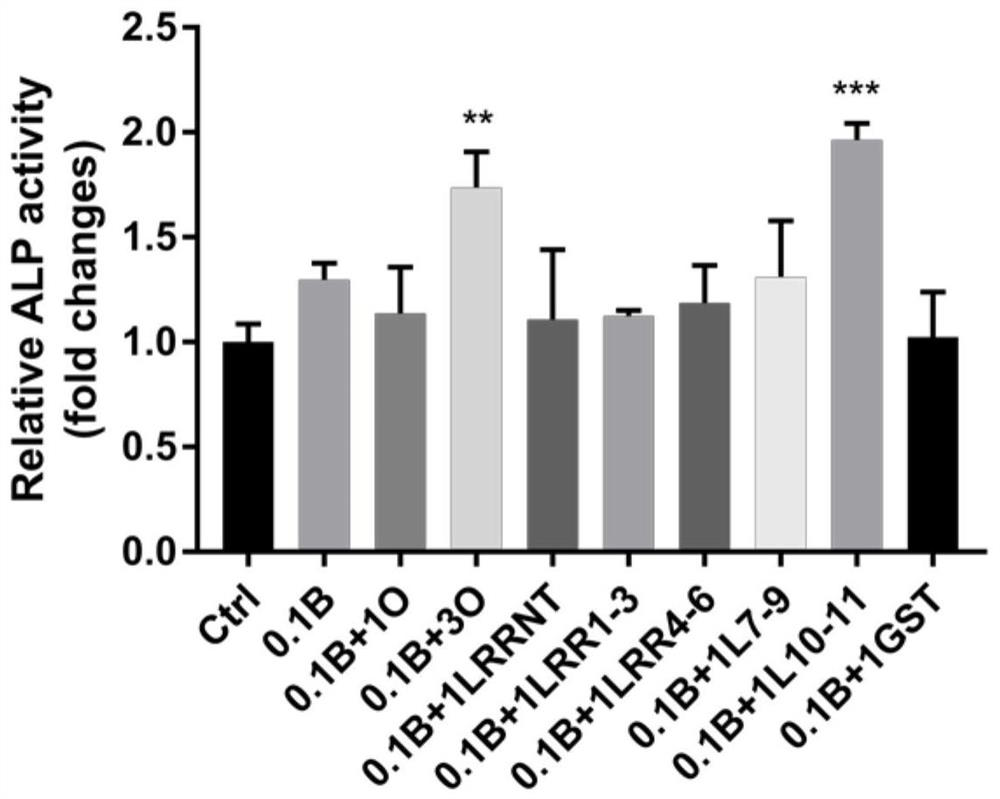
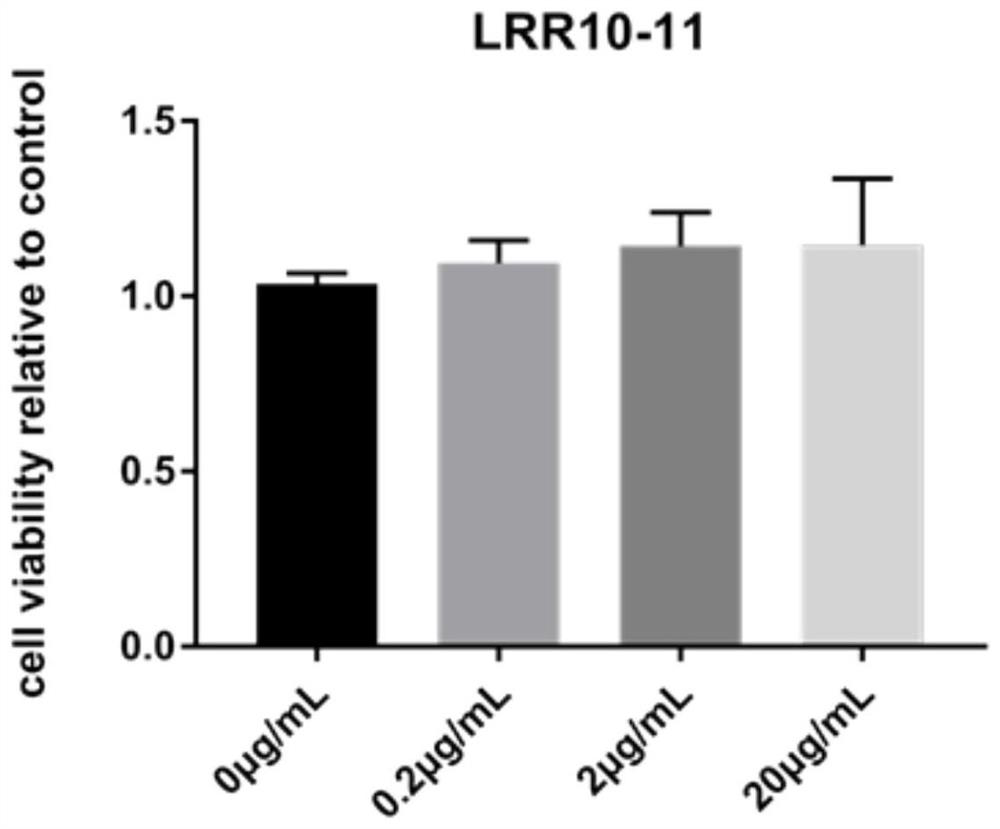
[0114] Embodiment 2 Alkaline phosphatase activity detection
[0115] Alkaline phosphatase (ALP) is a marker enzyme of mature osteoblasts and one of the markers of osteoblast differentiation. It can increase the concentration of local inorganic phosphoric acid and participate in the formation, metabolism and regeneration of calcified tissue. pNPP is a commonly used chromogenic substrate for phosphatase. Under alkaline conditions, it can generate para-nitrophenol under the action of alkaline phosphatase, which is a yellow product. The absorbance can be detected at 400-415nm. The darker the yellow product, the higher the alkaline phosphatase detection activity.
[0116] The steps of alkaline phosphatase (ALP) activity detection in this embodiment are:
[0117] (1) Inoculate hDPSCs into a 12-well plate, and add the following protein stimulation when the cells grow to 90% density. The groups are divided into: Ctrl group (0.1% BSA solution), 0.1B group (0.1 μg / mL BMP2 in 0.1% BSA...
Embodiment 3
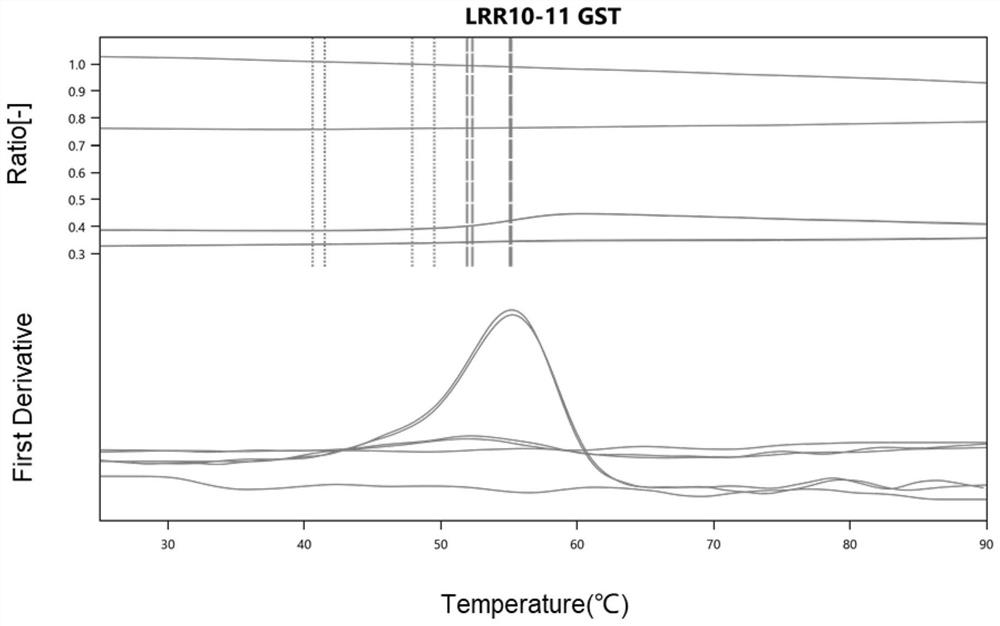
[0123] Example 3 OMD_LRR10-11 thermal denaturation assay
[0124] The protein unfolding transition temperature, Tm, refers to the temperature at which 50% of the protein unfolds. Proteins with high Tm are more stable because greater energy input is required to reach the transition temperature. Tm is an accurate and well-recognized measure of protein stability, so it is a parameter that must be determined. This example was used to measure the protein stability of the polypeptide OMD_LRR10-11.
[0125] The instrument used in this experiment was Prometheus (PR) (nanotemper, Germany).
[0126] The experimental steps are as follows: suck the polypeptide solution (without bubbles) obtained by the method of Example 1 with a certain concentration with a capillary glass tube, and then use Prometheus (PR) to measure Tm. The folded state of the protein is observed by detecting the change of the endogenous fluorescence of the protein. The fluorescence intensity of a single wavelength ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com