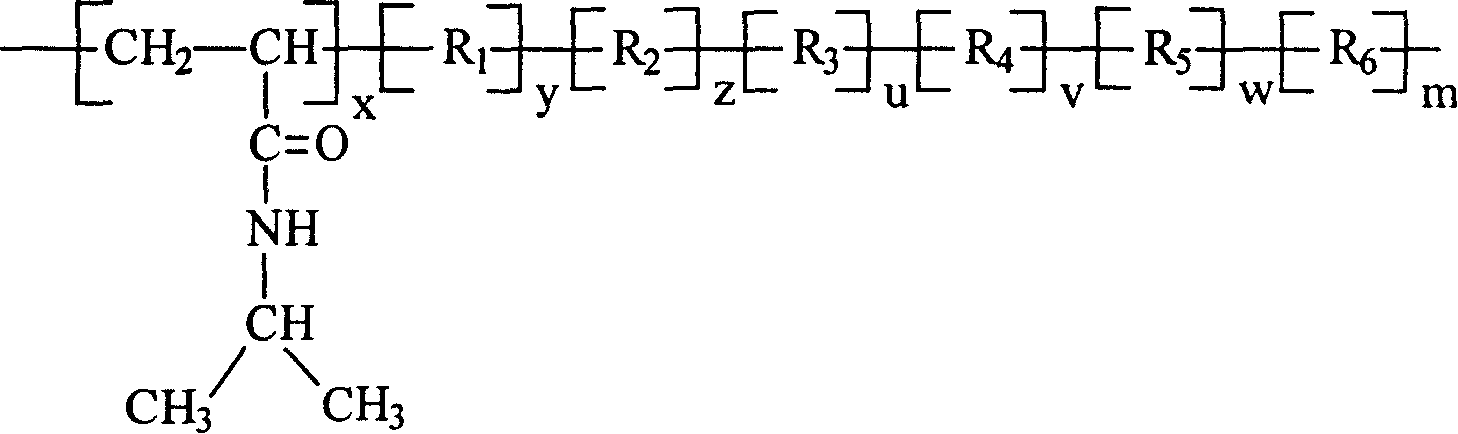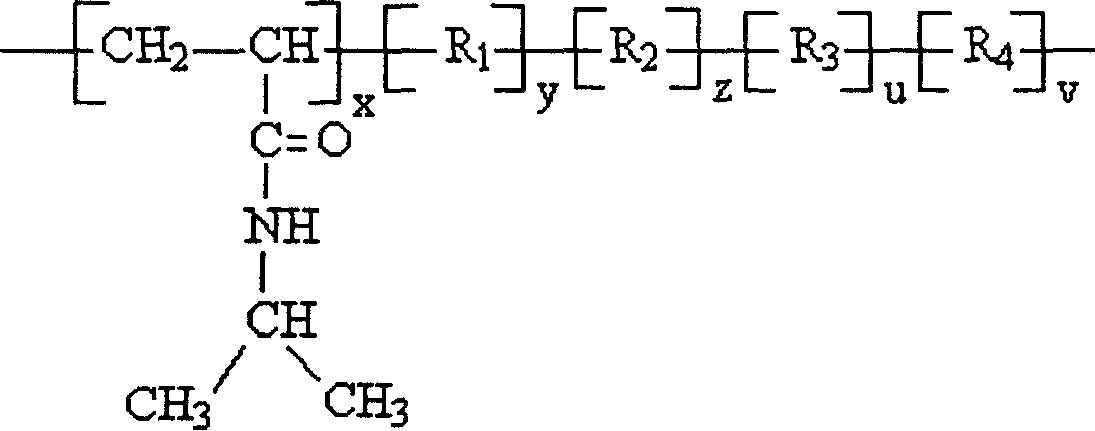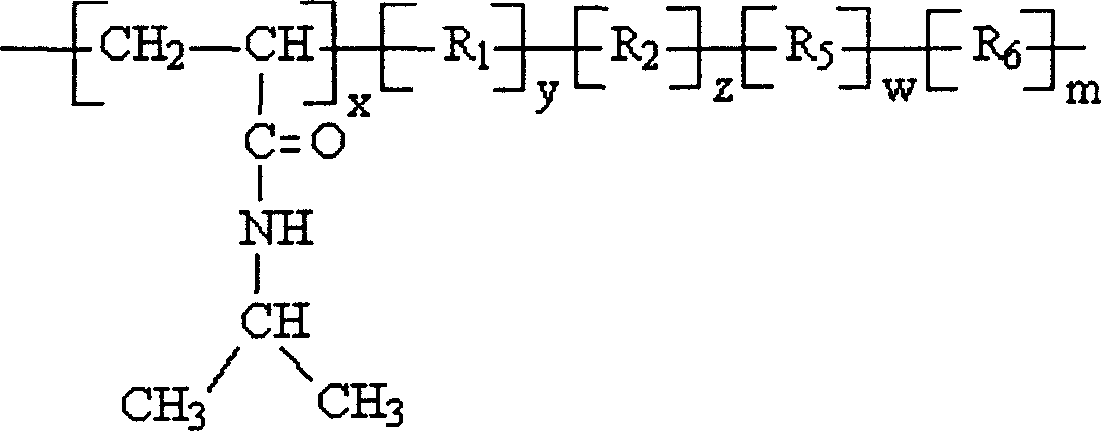Cholesterol ester-containing amphiphilic polymer sensitive to temperature and pH value and its prepn process
A technology of cholesteryl ester and pH, which is applied in the field of amphiphilic polymers and its preparation, can solve the problems of poor biocompatibility and achieve the effects of low synthesis cost, high yield, and good double sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment one: the synthesis of N-isopropylacrylamide / acrylic acid / cholesteryl acrylate copolymer: wherein R 1 with R 2 Same as cholesteryl acrylate, R 3 , R 4 , R 5 and R 6 Same, for acrylic.
[0021] 1. Synthesis of cholesteryl acrylate: the molar ratio of raw materials is cholesterol:acryloyl chloride=1:3.5. Dissolve 12.12g (0.0314mol) of cholesterol and 0.11mol of acryloyl chloride in 50ml of anhydrous benzene, add 0.15g of hydroquinone as a polymerization inhibitor, and heat to reflux for 7 hours. The reaction solution was dissolved in 70ml ether, followed by saturated Na 2 CO 3 solution, 10% HCl solution, washed with saturated NaCl solution; separated to collect the organic phase, anhydrous MgSO 4 Let dry overnight. Most of the solvent was evaporated by rotary evaporation, and the concentrated solution was redissolved in 30 ml of anhydrous ether, and then 300 ml of absolute ethanol was added for precipitation to obtain a white powdery solid with a yield ...
Embodiment 2
[0023] Embodiment two: the synthesis of N-isopropylacrylamide / acrylic acid 2-(N,N dimethylamino)ethyl ester / cholesteryl acrylate copolymer: R 1 with R 2 Same as cholesteryl acrylate, R 3 , R 4 , R 5 and R 6 Same, 2-(N,N dimethylamino)ethyl acrylate.
[0024] 1. Synthesis of cholesteryl acrylate: the molar ratio of raw materials is cholesterol:acryloyl chloride=1:5. Dissolve 12.12g (0.0314mol) of cholesterol and 0.157mol of acryloyl chloride in 50ml of anhydrous benzene, add 0.15g of hydroquinone as a polymerization inhibitor, and heat to reflux for 10 hours. The reaction solution was dissolved in 70ml ether, followed by saturated Na 2 CO 3 solution, 10% HCl solution, washed with saturated NaCl solution; separated to collect the organic phase, anhydrous MgSO 4 Let dry overnight. Most of the solvent was evaporated by rotary evaporation, and the concentrated solution was redissolved in a certain amount of 30ml of ether, and then 300ml of absolute ethanol was added for pr...
Embodiment 3
[0027] Embodiment three: the synthesis of N-isopropylacrylamide / 2-(N,N diethylamino)ethyl methacrylate / cholesteryl methacrylate copolymer: R 1 with R 2 Same as cholesteryl methacrylate, R 3 , R 4 , R 5 and R 6 Same, 2-(N,N diethylamino)ethyl methacrylate.
[0028] 1. Synthesis of cholesteryl methacrylate: The molar ratio of raw materials is cholesterol:methacryloyl chloride=1:4. Dissolve 12.12g (0.0314mol) of cholesterol and 0.126mol of methacryloyl chloride in 60ml of anhydrous benzene, add 0.2g of hydroquinone as a polymerization inhibitor, and heat to reflux for 8 hours. The reaction solution was dissolved in 60ml ether, followed by saturated Na 2 CO 3 solution, 10% HCl solution, washed with saturated NaCl solution; separated to collect the organic phase, anhydrous MgSO 4 Let dry overnight. Most of the solvent was evaporated by rotary evaporation, and the concentrated solution was redissolved in a certain amount of 30ml of ether, and then 300ml of absolute ethanol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com