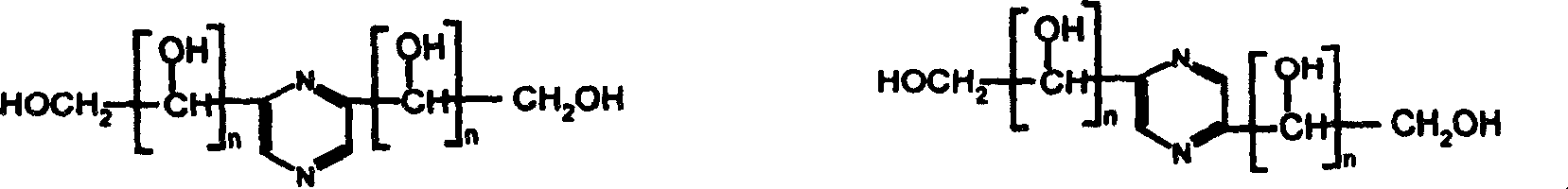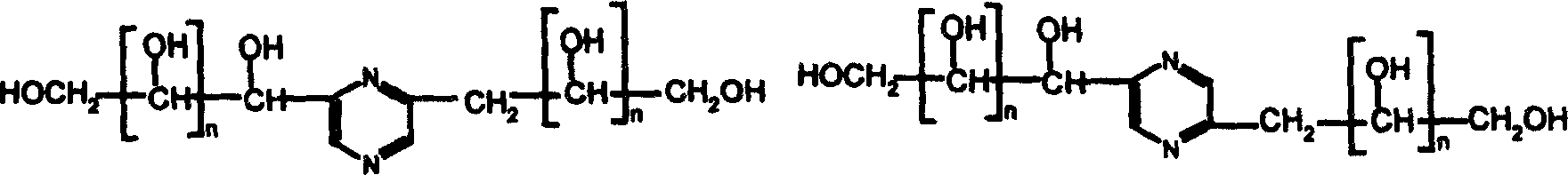Method for separation and purification of polyhydroxy-alkyl-pyrazine from suger amine reaction liquid
A technology of hydroxyalkylpyrazine and reaction solution, applied in the direction of organic chemistry, etc., can solve the problems of viscosity of the system, low concentration of polyhydroxyalkylpyrazine, low extraction efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
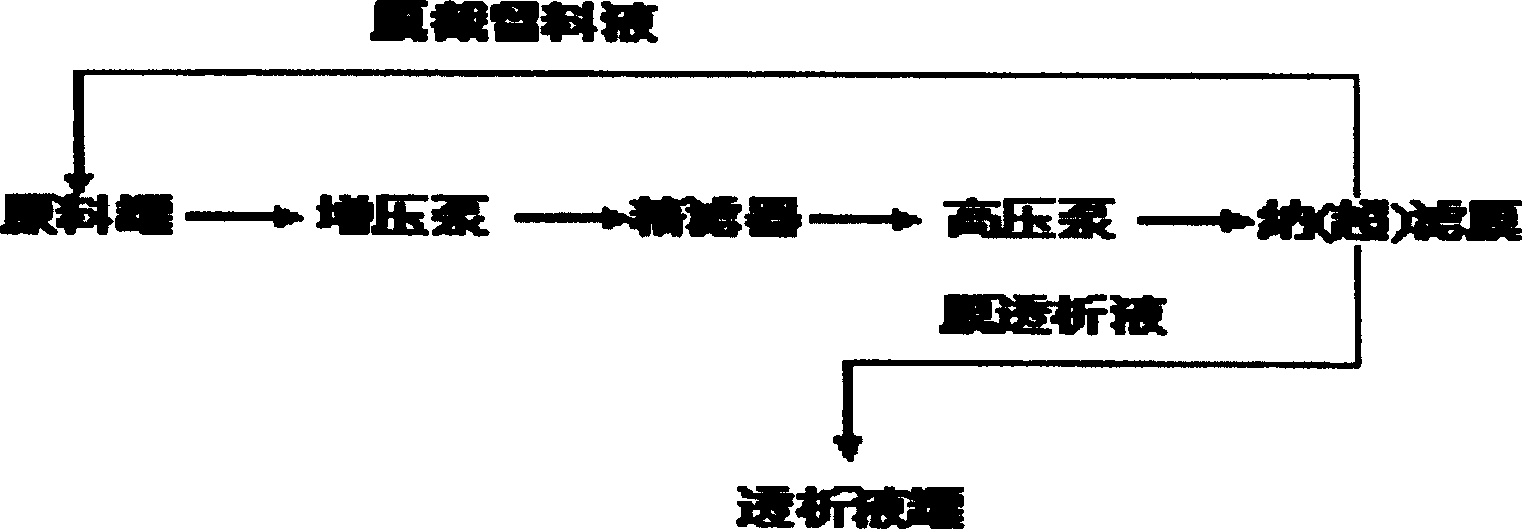
[0026] Separation and purification of 2,5-bis(1',2',3',4'-tetrahydroxybutyl)pyrazine from the reaction mixture of glucosamine and ammonia water by nanofiltration
[0027]20,000 grams of glucosamine hydrochloride was dissolved in 20,000 milliliters of 28% ammonia water, and stirred at room temperature for 21 days. Under the operating pressure of 3.5MPa, the mixture obtained by the reaction is passed through the tubular nanofiltration membrane module with a molecular weight cut-off of 500 from the raw material storage tank, 2,5-bis(1', 2', 3', 4' -Tetrahydroxybutyl) pyrazine aqueous solution is collected through the nanofiltration membrane to the permeate tank, and the macromolecular substances are intercepted by the membrane module and sent back to the raw material storage tank along with the feed liquid that has not passed through the membrane module, and then sent to the The membrane module is separated, and the separation is carried out in such a cycle. Membrane flux at 20L...
Embodiment 2
[0032] Separation and purification of the mixture of 2-hydroxymethyl-5-methylpyrazine and 2-hydroxymethyl-6-methylpyrazine from the reaction mixture of 1,3-dihydroxyacetone and ammonium acetate by ultrafiltration
[0033] 46 grams of 1,3-dihydroxyacetone was dissolved in absolute ethanol, added dropwise to 58 grams of ammonium acetate, and kept reflux during the dropwise addition, and refluxed for 3 hours after the dropwise addition. After the reaction, the reaction mixture was ultrafiltered in an ultrafiltration cup with an ultrafiltration membrane with a molecular weight cut-off of 1500, and compressed air was used as power to adjust the pressure to 0.3 MPa to collect the ultrafiltrate. This operating temperature is 20-30°C. In the later stage of ultrafiltration, add absolute ethanol repeatedly to dilute and continue ultrafiltration, 100 ml each time, until 2-hydroxymethyl-5-methylpyrazine and 2-hydroxymethyl-6-methylpyrazine in the ultrafiltrate are detected by GC-MS. The ...
Embodiment 3
[0036] Preliminary Purification of 2-(1', 2', 3', 4'-tetrahydroxybutyl)-5-(2", 3", 4" from the reaction mixture of glucose, fructose and diammonium hydrogen phosphate by ultrafiltration -trihydroxybutyl)-pyrazine and 2-(1′,2′,3′,4′-tetrahydroxybutyl)-6-(2″,3″,4″-trihydroxybutyl)-pyridine Zinc
[0037] 360 grams of glucose, 360 grams of fructose and 540 grams of diammonium hydrogen phosphate were dissolved in 2000 milliliters of water and reacted for 3 hours at 90-95°C. The mixture obtained by the reaction is passed through the hollow fiber ultrafiltration membrane module with a molecular weight cut off of 5000 from the raw material storage tank under pressure, the operating pressure is 0.3MPa, and the temperature is controlled at 30 to 40°C. Containing 2-(1', 2', 3', 4'-tetrahydroxybutyl)-5-(2", 3", 4"-trihydroxybutyl) pyrazine and 2-(1', 2' , 3′, 4′-tetrahydroxybutyl)-6-(2″, 3″, 4″-trihydroxybutyl) the aqueous solution of pyrazine is collected into permeate liquid tank thro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com