Phenyl yanthine derivatives
A technology of phenyl and purine, applied in the field of diol derivatives of xanthine, can solve the problems of limited efficacy and side effects of anti-inflammatory treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
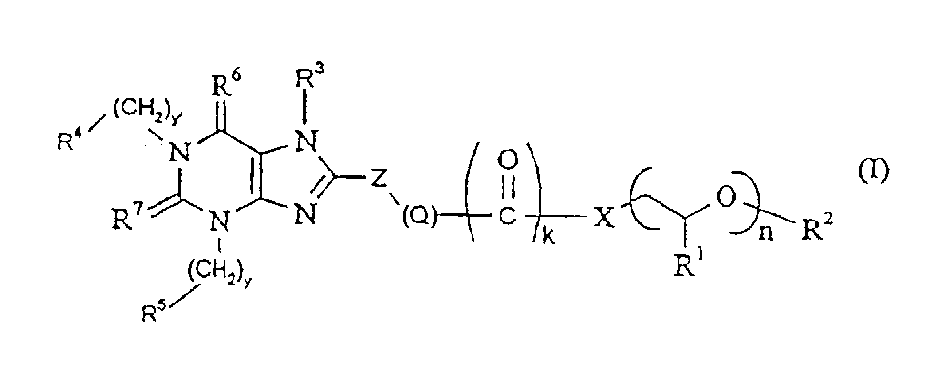
[0080] The temperature at which the process for preparing the compounds according to the present invention is carried out is generally from about -30°C to about 155°C, preferably from about -10°C to about 75°C. The preparation process is generally carried out under normal pressure. However, it is also possible to work under elevated or reduced pressure (for example in the range of 0.5-5 bar).
[0081] In the general formula (I) or (Ia), when X is oxygen or -NH-, by reacting with a reactive alkyl halide or acid halide in an inert solvent, preferably in the presence of a non-nucleophilic base, it can be import R 3 substituent, where R 3 As defined above. Solvents suitable for this process preferably include ethers such as diethyl ether, dioxane, tetrahydrofuran, ethylene glycol dimethyl ether; or hydrocarbons such as benzene, toluene, xylene, hexane, naphthenes or petroleum fractions; or Halogenated hydrocarbons such as dichloromethane, 1,2-dichloroethane, chloroform, carbon...
Embodiment 1
[0096] Example 1 Preparation of Compound 1 (E)-4-[1,3-bis(cyclohexylmethyl)-1,2,3,6-tetrahydro-2,6-dioxo-9H-purine-8 -yl]cinnamic acid (a) 1,3-bis(cyclohexylmethyl)urea
[0097] Under cooling, a mixture of cyclohexanemethylamine (Aldrich, 68.66g) and 5N sodium hydroxide (Fisher, 200ml) was vigorously stirred while a solution of phosgene (30.0g) in toluene (600ml) was added rapidly. After stirring for 20 minutes, the resulting mixture was filtered, and the precipitated solid was washed with water and dried (0.5 Torr) to give 1,3-bis(cyclohexylmethyl)urea (72.72 g, 95%) as a white powder, m.p.150 -152°C; 1 H-NMR (DMSO-d 6 )δ:5.74(brt,J=5.8Hz,2,2NH),2.81(t,J=6.3Hz,4,2NCH 2 ), 1.62, 1.25 and 0.85 (all m, 22, 2 cyclohexyl).
[0098] C 15 h 28 N 2 Anal. Calcd. for O: C, 71.38; H, 11.18; N, 11.10. Found: C, 71.22; H, 11.17; N, 11.15. (b) 6-Amino-1,3-bis(cyclohexylmethyl)uracil
[0099]Cyanoacetic acid (Aldrich, 21.0 g) was dissolved in acetic anhydride (260 ml). This solut...
Embodiment 34
[0107] Example 34 (E)-4-[1,3-bis(benzyl)-1,2,3,6-tetrahydro-2,6-dioxo-9H-purin-8-yl]cinnamic acid nine Ethylene glycol methyl ether ester (a) nonaethylene glycol monomethyl ether
[0108] Sodium hydride (8.6 g, 344 mmol 95%) was added to a solution of hexaethylene glycol (Aldrich, 100 g) in anhydrous tetrahydrofuran (1000 ml) at 15°C. The resulting mixture was stirred while allowing to reach ambient temperature over 1 hour. Benzyl bromide (Aldrich, 59.9 g) was added dropwise over 1 hour and the resulting mixture was stirred at ambient temperature for 16 hours. The cooled mixture was diluted with water (200ml) and extracted with ether. The combined ether extracts were washed with water. The combined aqueous layers were saturated with sodium chloride and extracted with dichloromethane. The combined dichloromethane layers were washed with saturated sodium chloride and dried (magnesium sulfate). The volatiles were removed under reduced pressure, leaving hexaethylene glycol mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com