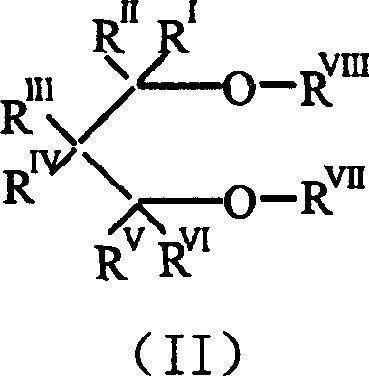
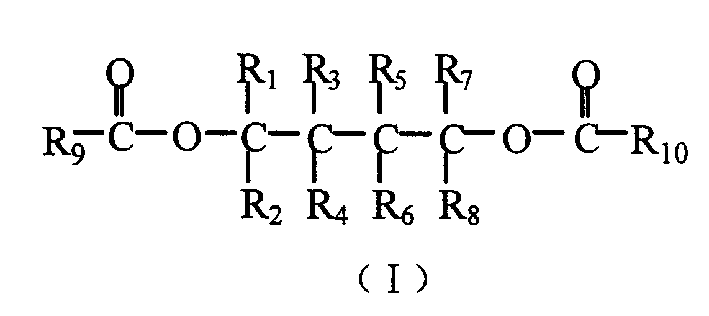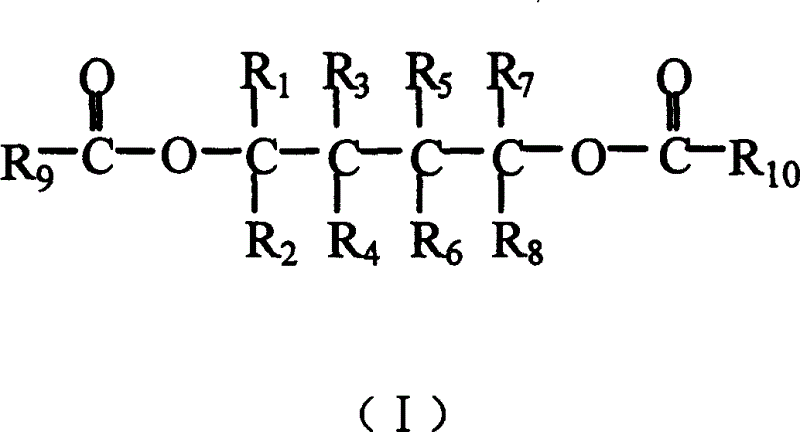Catalyst component for alkene polyreaction and its catalyst
An olefin polymerization and catalyst technology, which is applied in the field of catalyst components and catalysts for olefin polymerization, can solve the problems of narrow polymer molecular weight distribution, unfavorable polymer development, and low catalyst catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Synthesis of 2,3-diisopropyl-1,4-butanediol dibenzoate
[0051] 1) Synthesis of 2,3-diisopropyl-1,4-butanediol
[0052] The mixture of 5.1 g of lithium aluminum hydride and 120 ml of diethyl ether was cooled to 0°C, and at this temperature, a mixture of 11 g of diethyl 2,3-diisopropyl-1,4-succinate and 60 ml of diethyl ether was slowly added dropwise. After the addition, the temperature was raised to reflux and maintained at reflux for 1 hour. Then, it was lowered to 0°C, and 5ml of 15% sodium hydroxide solution and 20ml of water were added dropwise. After rising to room temperature, react for half an hour. After filtering, washing, drying and concentrating the organic phase, 8.4 g of product (76%) was obtained by distillation under reduced pressure.
[0053] bp118℃ / 0.1mmHg; 1 H-NMR (δ, ppm, CDCl 3 ), 0.9(14H), 1.4(2H), 1.9(4H) and 3.7(2H).
[0054] 2) Synthesis of 2,3-diisopropyl-1,4-butanediol dibenzoate
[0055] 7.7 g of 2,3-diisopropyl-1,4-butanedio...
Embodiment 2
[0057] Example 2 2,3-Dimethyl-1,4-butanediol dibenzoate
[0058] According to a method similar to Example 1, 1) 2,3-dimethyl-1,4-butanediol bp95°C / 0.1mmHg was prepared; 1 H-NMR (δ, ppm, CDCl 3 ), 0.7-1.8(8H), 3.2-3.8(4H) and 4.8(2H).
[0059] 2) 2,3-Dimethyl-1,4-butanediol dibenzoate
[0060] 1 H-NMR (δ, ppm, CDCl 3 ), 1.1-1.6(8H), 5.0-5.5(4H), 7.3-8.2(10H).
Embodiment 3
[0061] Example 3 Preparation of 2,5-dimethyl-2,5-hexanediol dibenzoate
[0062] Add 30ml tetrahydrofuran and 0.09mol pyridine to 0.03mol 2,5-dimethyl-2,5-hexanediol, add 0.075mol benzoyl chloride under stirring, and heat to reflux for 4h. After cooling, add 20ml of saturated brine, extract with ethyl acetate, anhydrous Na 2 SO 4 Dry and remove solvent. Column chromatography gave 2,5-dimethyl-2,5-hexanediol dibenzoate as a colorless viscous liquid with a yield of 93%. 1 HNMR (TMS, CDCl 3 , ppm): δ1.6 (12H, s, methyl H), 2.0 (4H, s, methylene H), 7.4-8.0 (10H, m, benzene ring H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com