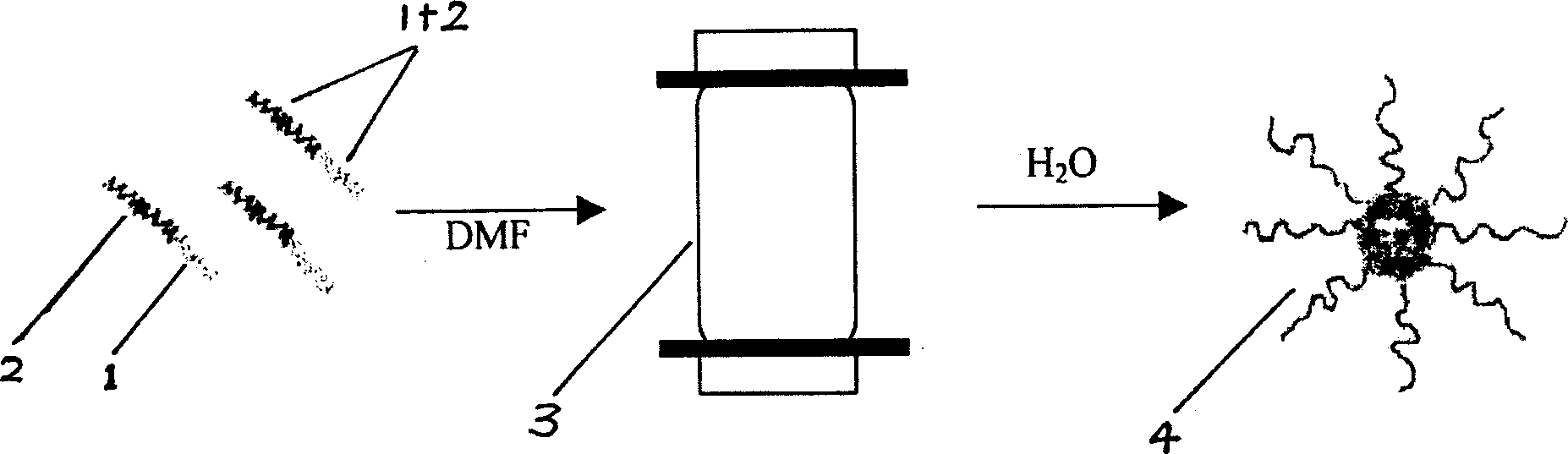
Nano micro granules, their preparation and medicinal uses of campotothecin derivative
A nanoparticle and camptothecin technology, which is applied in the direction of drug combination, antineoplastic drugs, freeze-dried delivery, etc., can solve the problems of short half-life, low tissue affinity, unstable preparation quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
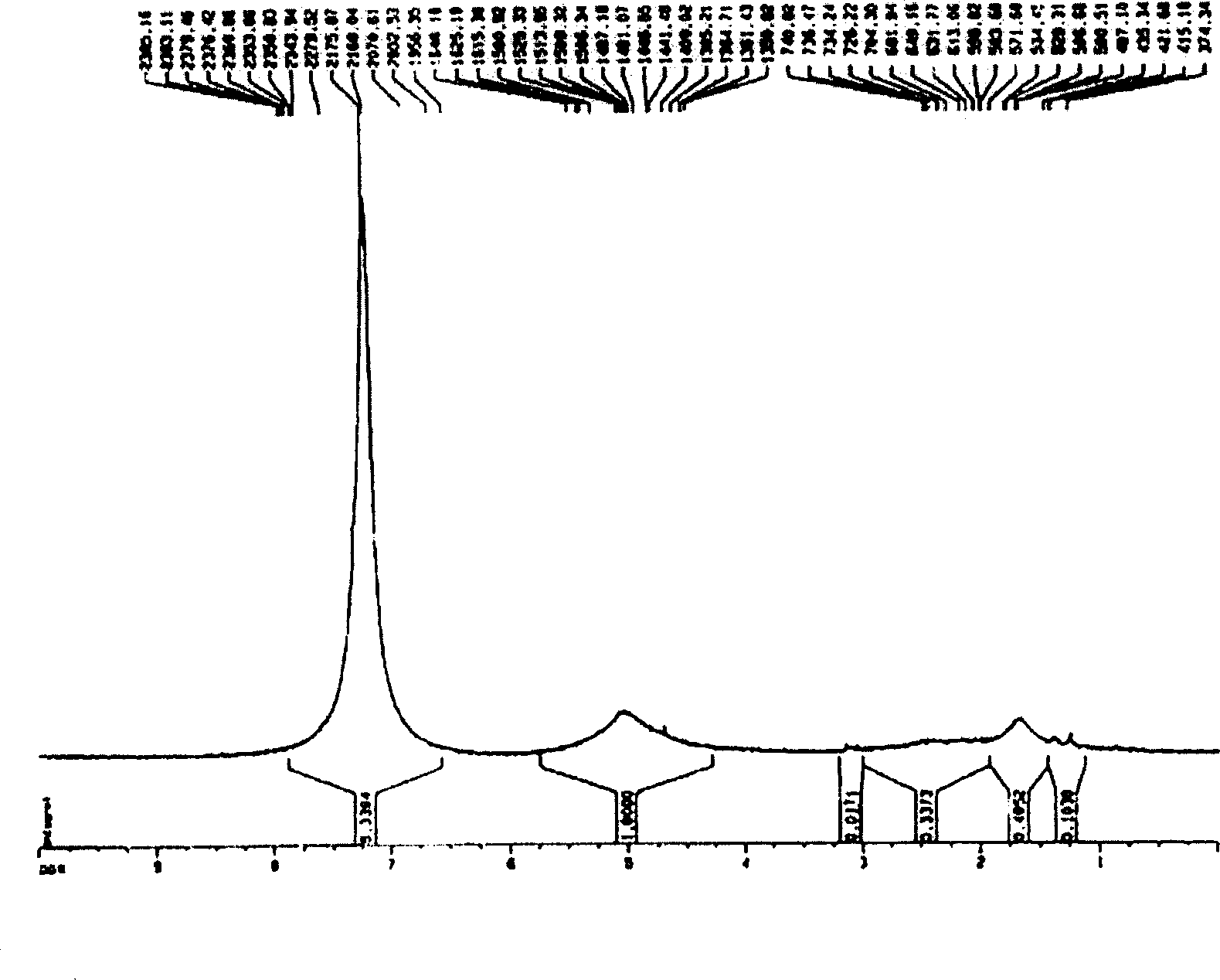
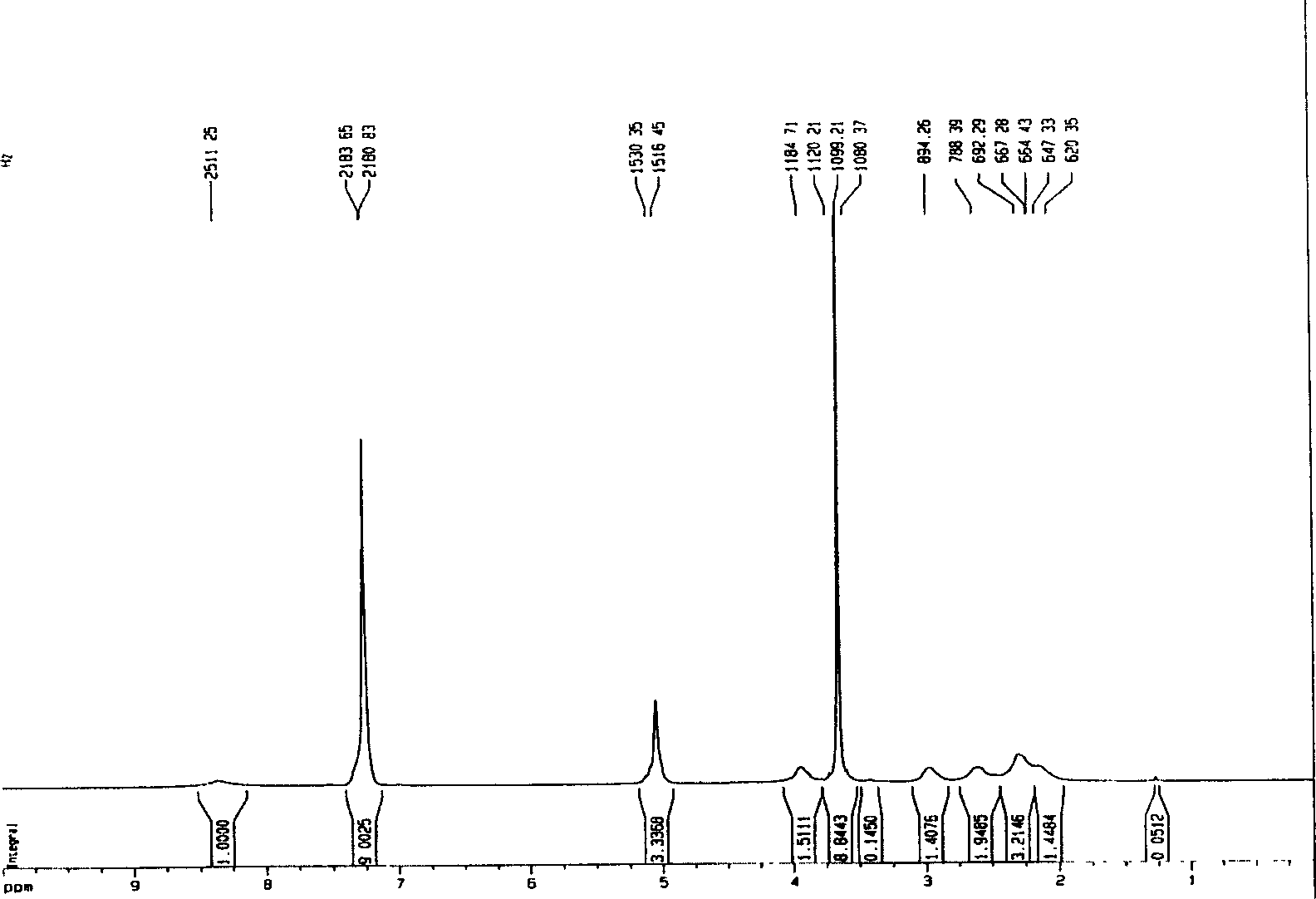
Image
Examples
Embodiment 1
[0093] Example 1 ω-amino-terminated polyethylene glycol (MeO-PEG-NH 2 )Synthesis
[0094] experiment procedure
[0095] Take 40g of MeO-PEG, vacuum dehydrate at 120℃ for 5h, cool at room temperature, add 200ml CH 2 Cl 2 Dissolved, then added a double excess of p-toluenesulfonyl chloride / pyridine solution (5% w / v), and reacted at room temperature for 24 hours in the dark. Add 431ml of 3M HCl to the reaction solution, mix well to neutralize it with pyridine, put all the liquid in a separatory funnel, let stand to separate layers, take out the lower organic layer, and use solid NaHCO 3 Wash, filter, vacuum rotary evaporate CH 2 Cl 2 , adding 20ml tetrahydrofuran (THF) to dissolve, a large amount of diethyl ether was precipitated, filtered, and vacuum-dried to obtain PEG-p-toluenesulfonyl salt in the form of white powder with a yield of 79%. 10g of PEG-p-toluenesulfonyl salt and excess concentrated ammonia water (28%) were sealed and reacted at 120°C for 8h, cool...
Embodiment 2
[0096] The synthesis of embodiment 2 PEG-PBLG block copolymer
[0097] Stir and mix 96g of L-glutamic acid and 78g of benzyl alcohol, add dropwise 106g of 60% concentrated sulfuric acid, make the solution clear after 20min, and react under vacuum at 70°C for 4h. The reaction is poured into 800ml containing NaHCO in an equimolar amount with concentrated sulfuric acid 3 In the aqueous solution, stir to produce white foamy precipitate, filter, recrystallize with 2000ml 5% ethanol 70 ℃ hot water, stand overnight at 4 ℃, filter and wash to get the product L-glutamic acid γ-benzyl ester (BLG) 80g, silver-white lamellae, yield 83.3%. The melting point is 172-174°C. Suspend 40g of BLG in a closed container of 160ml of THF, add 26g of triphosgene, stir and react at 60-65°C for 10min, the reaction solution is clear and transparent, react for another 20min, pour the reaction solution into 500ml of petroleum ether for precipitation, -20°C Stand for 48 hours, filter, wash,...
Embodiment 3
[0099] The synthesis of embodiment 3 PEG-PBLG block copolymer
[0100] The method for synthesizing BLG-NCA monomer with the method for embodiment 2. Then the BLG-NCA monomer (A) 2g that will be synthesized is dissolved in 40ml benzene-dioxane mixed solvent, is initiator with triethylamine, respectively by monomer and initiator mol ratio (A / I) 50 / 1 was added to the initiator, and the reaction was stirred at room temperature for 72h. At the beginning of the reaction there is CO 2 Bubbles are generated, and the viscosity of the reaction solution gradually increases. The reaction solution is poured into 20 times of absolute ethanol to precipitate, filtered, and vacuum-dried to obtain a PEG-PBLG block copolymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com