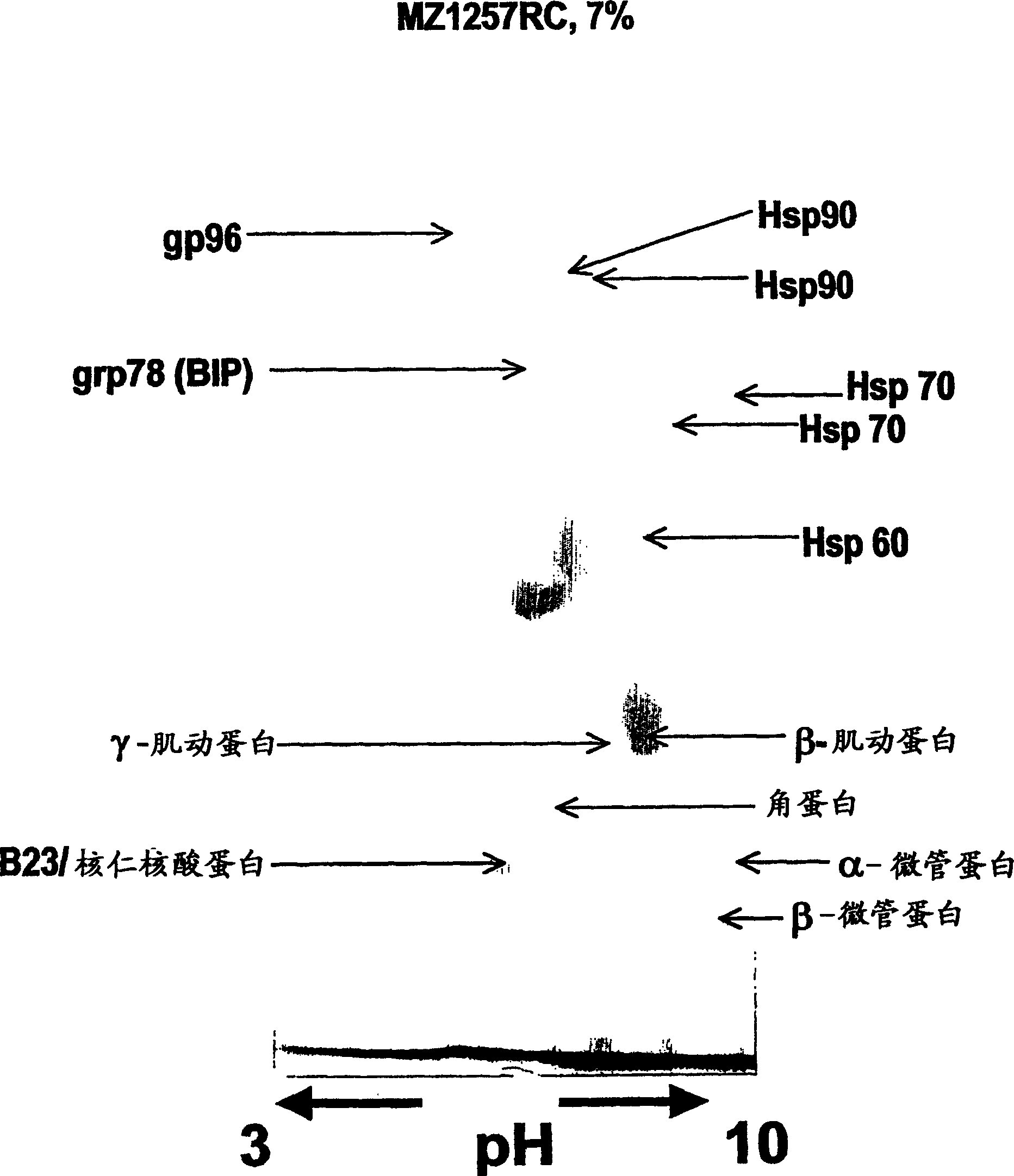
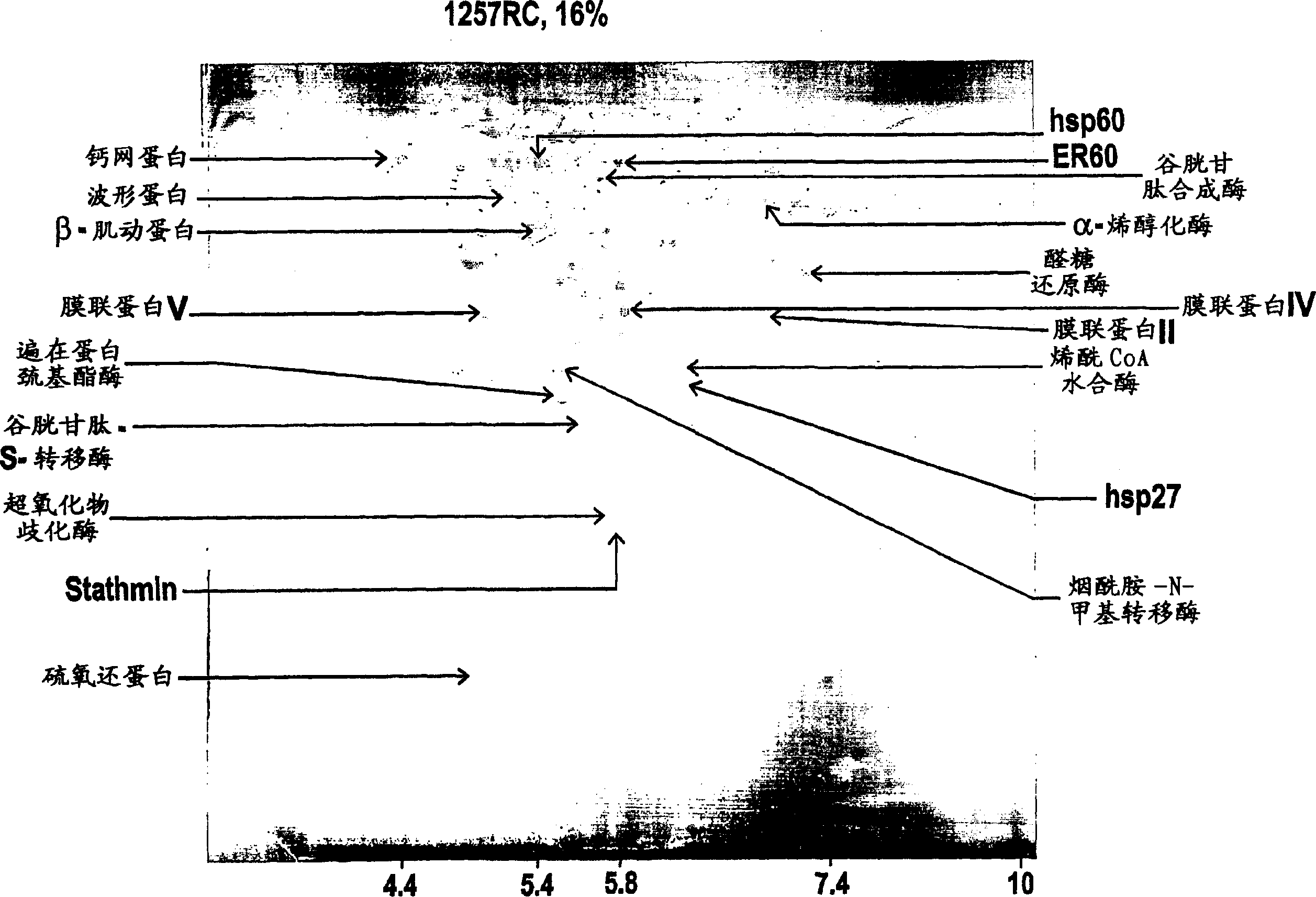
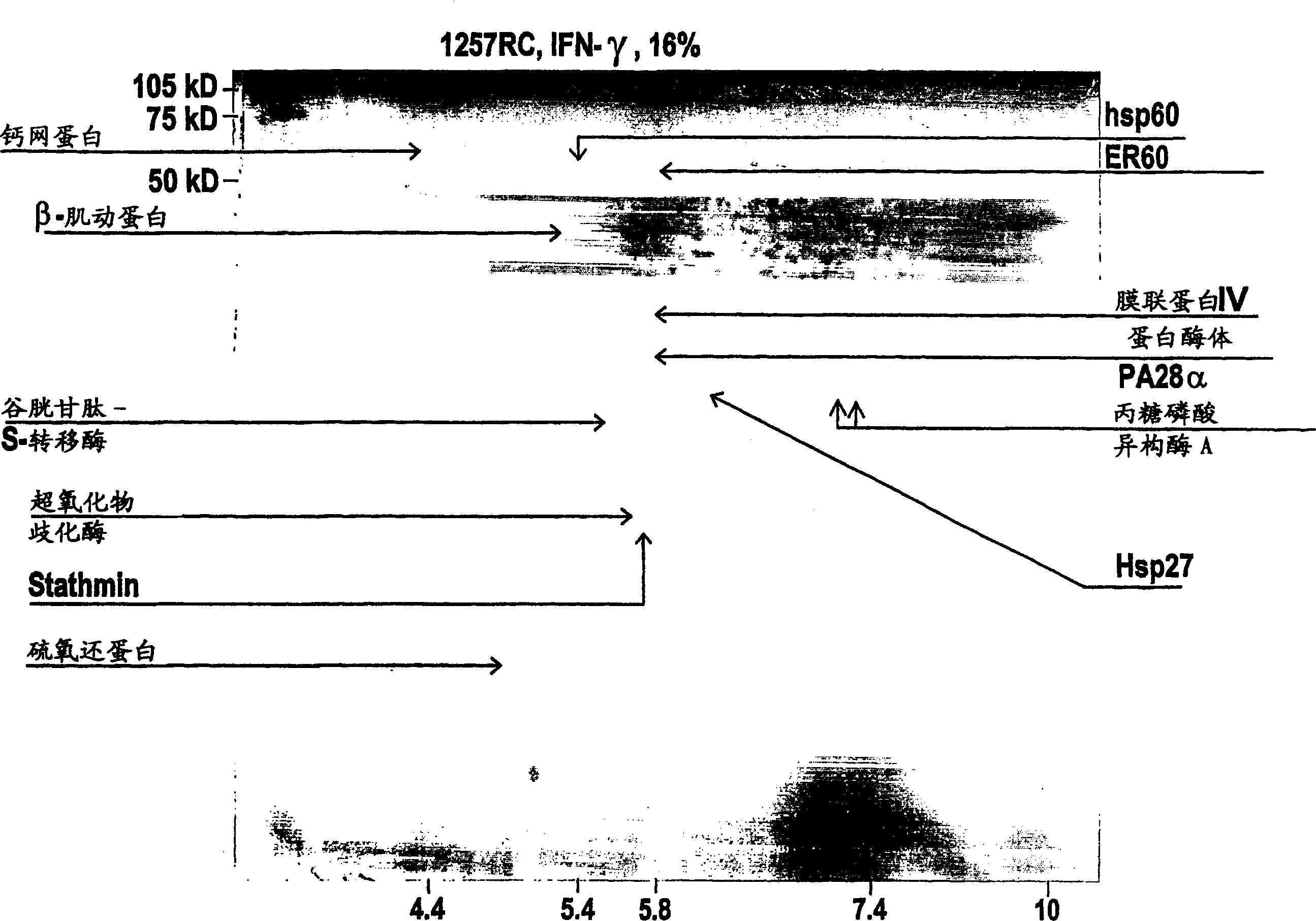
Renal cell carcinoma markers
A tumor marker, cytokeratin technology, applied in the field of preparing antibodies and antibody fusion proteins targeting tumor markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Cell culture and IFN-γ treatment
[0107] MZ1257RC and MZ1940RC represent well-defined human cell lines identified as clear cell types of renal cell carcinoma (RCC) (Seliger, B. et al., Cancer Res. 1996, Vol. 56, pp. 1756-60), while their corresponding The normal kidney tissues of MZ2733RC and MZ2733NN were recently established from patients with primary RCC clear cell type. All RCC cell lines were maintained in DMEM supplemented with 10% fetal calf serum, 2 mM glutamine and 100 U / ml penicillin / 100 μg / ml streptomycin.
[0108] MZ1257RC cells were treated with IFN-γ for 48 hours in the presence of 300 U / ml recombinant IFN-γ (Imukin, Boehringer Ingelheim, Ingelheim, Germany).
[0109] serum sample :
[0110] All serum samples were isolated from human venous blood samples collected from patients diagnosed with renal cell carcinoma or from normal voluntary donors with the consent of each individual.
Embodiment 2
[0112] two-dimensional gel electrophoresis
[0113] Sample Preparation:
[0114] Cell lines expanded to 5 x 10 cells per batch 7 to 1×10 8 , and then harvested by trypsination. The cell pellet was washed 3-4 times in phosphate buffered saline (PBS), and then stored in a sterile cryogenic tube, and the dried cell pellet was 5×10 6 or 1×10 7 Aliquots of cells / tubes were stored in liquid nitrogen until use. In lysis buffer (7M urea, 2M thiourea, 0.2M dimethyl benzyl ammonium propanesulfonate (NDSB), 1% dithiothreitol (DTT), 4% 3-[(3-cholamidopropyl) dimethyl Base-ammino]-1-propane-sulfonate (CHAPS), 0.5% pharmalytes and a small amount of dye bromophenol blue) to resuspend the cell pellet. Lysates were sonicated (3 x 4 minutes in an ultrasonic bath) and then clarified by centrifugation in a microcentrifuge (90 min, 15°C, 13,000 rpm).
[0115] protein quantification
[0116] Protein quantification was performed according to the experimental protocol described by Ramag...
Embodiment 3
[0124] Western blot:
[0125] For immunoblot analysis, colloidal Coomassie blue prestained 2-D PAGE gels were printed on ImmobilonP membranes using the ISO-DALT tank blotting system (Amersham Pharmacia Biotech), and SDS-PAGE electrophoresis buffer supplemented with 20% methanol As transfer buffer, and 500 volt hours per transfer. Then the blot was incubated in blocking solution (140mM NaCl, 10mM Tris / HCl pH7.4, 0.4% Tween20, 5% low-fat milk powder and 10% horse serum) for 1 hour, in Tris buffered saline (TBS: 140mM NaCl, 10mM Tris / HCl HCl pH7.4), then incubated overnight at 4°C with control or patient serum diluted 1:20 in antibody incubation buffer (TBS, 0.1% Tween20, 2% low-fat dry milk). Then wash the membrane 3 times in TBS, 0.4% Tween20 (10 minutes each time), and mix with horseradish peroxidase (HRP)-conjugated second monoclonal antibody solution (20ml / membrane, rabbit anti-human IgG) at room temperature , diluted 1:1000 in antibody incubation buffer) and incubated f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com