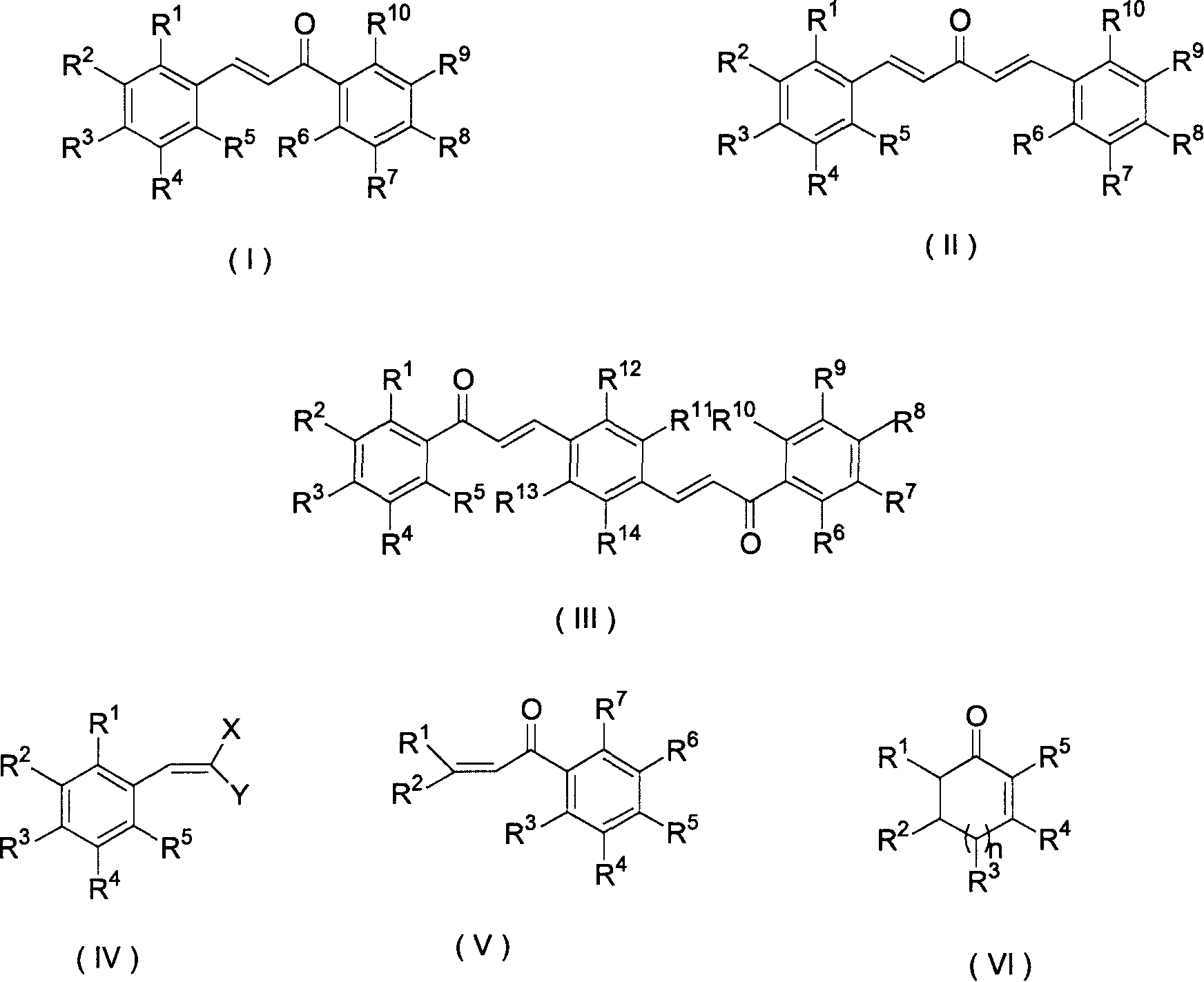
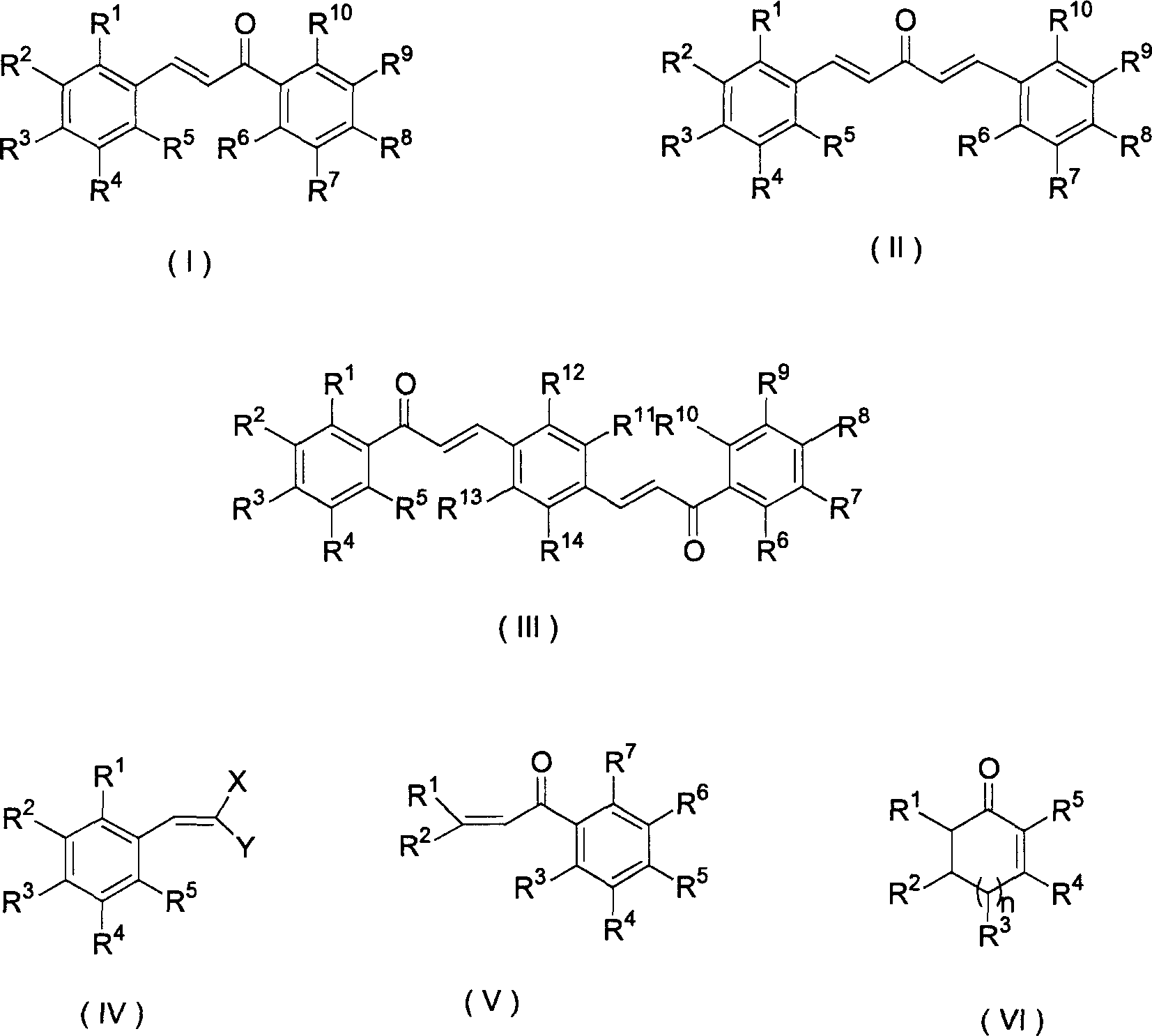
Epoxide generation method by catalytic oxidation of functionalized alkene
A technology of epoxide and oxidation function, which is applied in the direction of organic chemical methods, chemical instruments and methods, organic oxidation, etc., can solve the problems of high price of peroxyacid, low conversion rate of raw materials, long reaction time, etc., and achieve low cost, High reaction efficiency and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of solid base: Dissolve 58 grams of potassium fluoride in 100 grams of water, add 100 grams of neutral alumina (for chromatography, 100-200 mesh), stir and react at 60°C for 1 hour, then heat up and evaporate the water to dryness, solid The product was dried at 120°C for 4 hours to obtain the solid base KF / Al 2 o 3 , placed in a desiccator for later use.
Embodiment 2
[0029] In a 250ml three-necked flask, add 25mL of N,N-dimethylformamide, 2.325 grams (10mmol) of trichloroisocyanuric acid, 2.0 grams of KF / Al 2 o 3 , after stirring for 15min, add chalcone (i.e. R in the structure (I) 1 ~R 10 hydrogen) 2.08 grams (10mmol), stirred at room temperature for 10min, filtered to remove KF / Al 2 o 3 Solid, most of the solvent was evaporated under reduced pressure, then water (10 mL) was added to the residue, and extracted with dichloromethane (2 × 20 mL) (note: the extracted water can be used in the next batch of reactions, without affecting the yield and purity of the reaction). After the extract was dried over anhydrous sodium sulfate, the solvent was evaporated to obtain an epoxidized product, which was recrystallized, dried, and weighed to obtain 2.021 grams, with a yield of 90.1% (calculated as olefins), and an HPLC analysis purity of 99%. above. The content determination adopts Waters high-performance liquid chromatography system, includi...
Embodiment 3
[0031] The organic solvent used is a mixture of benzene and N,N-dimethylformamide. The test method and steps are the same as in Example 2. The reaction time is 30 minutes, the yield is 78.2%, and the HPLC analysis purity is above 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com