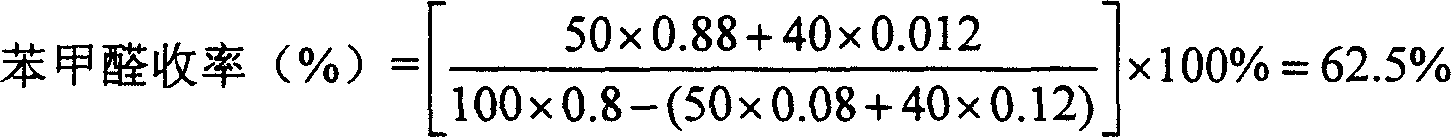Process for preparing natural benzaldehyde
A benzaldehyde, natural technology, applied in the field of benzaldehyde, can solve the problems of difficult selection of a non-toxic phase transfer catalyst, lower naturalness of product benzaldehyde, immature industrialization conditions, etc., to avoid emulsification and increase reaction rate , The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Take by weighing 1.2g of NaOH, 0.3g of Tween-40, and 210g of water, add to a four-necked flask and stir evenly, then add 40g of Guangxi cinnamon oil (containing cinnamaldehyde 80%) under stirring, and stir at a slow speed The temperature of the system was raised to 98° C., and the reaction was continuously stirred for 24 hours under reflux. Finally, the water phase and the oil phase were separated, and the content of benzaldehyde and cinnamaldehyde in the oil phase was analyzed by gas chromatography, and the yield of benzaldehyde was calculated to be 44%.
[0026] Adopt the technique of the present invention below, so that contrast with above-mentioned result.
[0027] Weigh ethanolamine 100g, Na 2 CO 3 100g (for analytically pure anhydrous matter), 5g of dodecyltrimethylammonium bromide, add in 10Kg water phase and mix uniformly; The above mixed aqueous solution; add 1.85 kg of cinnamon oil containing cinnamaldehyde 80% to the dropping funnel, and cover a...
Embodiment 2
[0029] Embodiment 2: with 2.5gNa 2 CO 3 Replace the 1.2gNaOH in the embodiment 1, all the other are the same as the embodiment 1, finally the yield of benzaldehyde is 48.3%.
[0030] Likewise, the process of the present invention was adopted in order to compare with the above-mentioned results.
[0031] Similar to Example 1, the difference is that the organic base adopts diethanolamine 100g, Na 2 CO 3120g, cationic surfactant adopts tetradecyltrimethylammonium bromide 2g and octadecyltrimethylammonium bromide 2g, water is 10.5kg, cinnamon oil is 1.2kg, reaction temperature is 102 ℃, cinnamon oil The instillation time was controlled at 8 hours. The yield of benzaldehyde was 57.5%.
Embodiment 3
[0032] Embodiment 3: similar to Example 1, the difference is that each 0.08g of dodecyltrimethylammonium bromide, tetradecyltrimethylammonium bromide, cetyltrimethylammonium bromide Replace the Tween-40 in embodiment 1, all the other are with embodiment 1, the yield that finally gets benzaldehyde is 49.0% (dodecyl trimethyl ammonium bromide), 49.2% (tetradecyl trimethyl ammonium bromide) ammonium bromide), 49.5% (hexadecyltrimethylammonium bromide).
[0033] Likewise, the process of the present invention was adopted in order to compare with the above-mentioned results.
[0034] Similar to Example 1, the difference is that the organic base adopts diethanolamine 60g, triethanolamine 40g, Na 2 CO 3 150g, the cationic surfactant adopts 1g of dodecyltrimethylammonium bromide, 16kg of water, 1kg of cinnamon oil, the reaction temperature is 105°C, and the dripping time of cinnamon oil is controlled at 6 hours. The yield of benzaldehyde was 56.4%. When the organic base adopts etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com