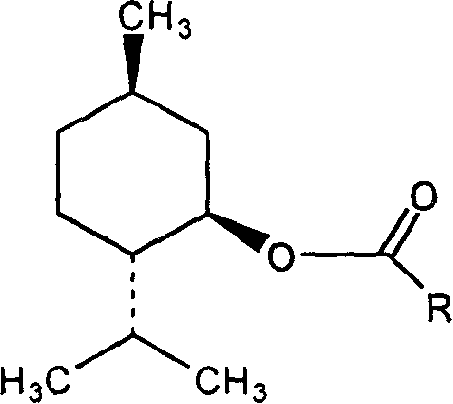
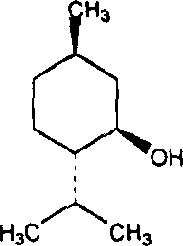
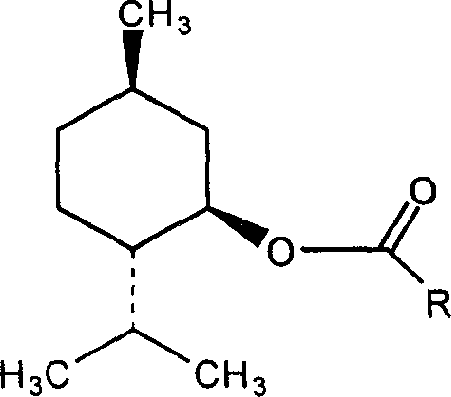
Menthol derivative containing fatty acid and preparation containing said derivative
A technology of menthol derivatives and fatty acids, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: Menthyl laurate (ML)
[0014] Take 5g of menthol and add 50mL of dichloromethane to dissolve, add lauroyl chloride in an equimolar amount, mix well, add 0.5ml of pyridine, react at 30°C for 5 hours, distill off the dichloromethane, add 10ml of water to the resultant, React at 30°C for 0.5 hours to eliminate unreacted lauroyl chloride, add 100ml of water, mix well, extract with dichloromethane three times in total, 30ml each time, combine dichloromethane, use anhydrous sodium sulfate to remove residual water, reduce Dichloromethane was removed by pressure steaming to obtain the product.
[0015] IR (KBr tablet) v / cm -1 : Characteristic peaks 1735.1 (ester, C=O), 1162.5 (C-O), 1112.8 (C-O). 13 C NMR (DMSO-d 6 ), δ: 172 (ester, C=O). ESI-MS m / z: 339.6[M] + .
Embodiment 2
[0016] Embodiment 2: Menthyl myristate (MM)
[0017] Take 5 g of menthol and add 50 mL of toluene to dissolve it, add myristoyl chloride in an equimolar amount, mix well, add 0.3 g of lutidine (DMAP), reflux at 60°C for 3 hours, distill off the toluene, and Add 5ml of water, react at 60°C for 0.5 hours, eliminate unreacted myristoyl chloride, add 100ml of water, mix well, extract with ethyl acetate, a total of three times, 30ml each time, combine the ethyl acetate extracts, wash with anhydrous sulfuric acid Sodium was removed to remove residual moisture, and ethyl acetate was evaporated under reduced pressure to obtain the product.
[0018] IR (KBr tablet) v / cm -1 : Characteristic peaks 1735.0 (ester, C=O), 1160.2 (C-O), 1113.5 (C-O). 13 C NMR (DMSO-d 6 ), δ: 172 (ester, C=O). ESI-MS m / z: 367.6[M] + . proved to be the target compound.
Embodiment 3
[0019] Embodiment 3: menthol oleate (MO)
[0020] Take 5 g of menthol and add 50 mL of isopropyl ether to dissolve, add oleic acid chloride at a molar ratio of 1:1.1, mix well, add 1 ml of triethylamine, reflux at 40°C for 7 hours, distill off the isopropyl ether, and obtain Add 5ml of water, react at 60°C for 0.5 hours, eliminate unreacted oleic acid chloride, add 100ml of water, mix well, extract with dichloromethane, a total of three times, 30ml each time, combine the dichloromethane extracts, and use anhydrous Sodium sulfate was used to remove the residual moisture, and dichloromethane was distilled off under reduced pressure to obtain the product.
[0021] IR (KBr tablet) v / cm -1 : Characteristic peaks 1735.41 (ester, C=O), 1160.8 (C-O), 1112.5 (C-O). 13 C NMR (DMSO-d 6 ), δ: 172 (ester, C=O). ESI-MS m / z: 421.7[M] + . proved to be the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com