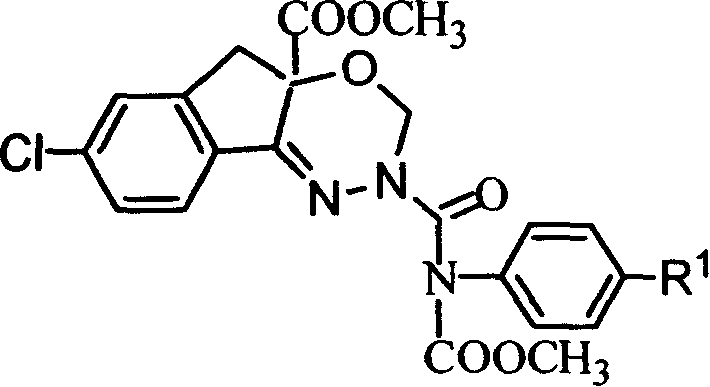
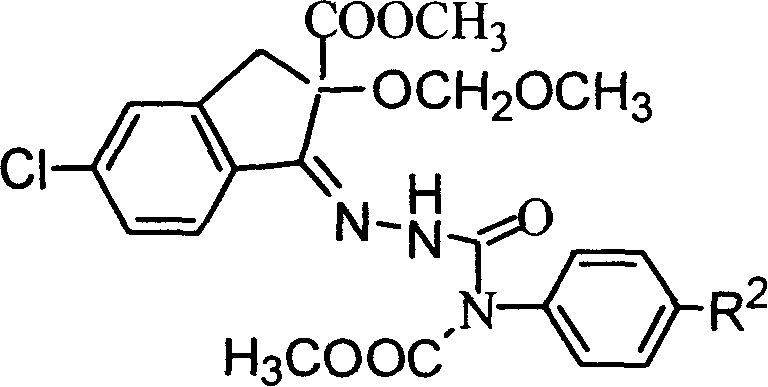
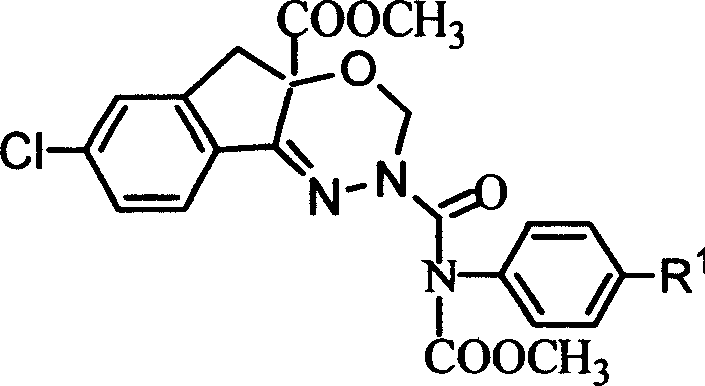
Pesticide and its preparing method
A technology of insecticide and insecticidal activity, applied in the field of insecticide and its preparation of fused ring derivatives, which can solve the problems of paralysis of target pests, loss of function of nerve cells, poor coordination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 3-(5-chloro-2-carboxyphenyl)propionic acid (steps a, b, c)
[0039] In a 500mL three-neck flask, add 40g of p-chlorophenylacetic acid and 140mL of dichloroethane, raise the temperature to reflux, add 30mL of thionyl chloride dropwise, and react under reflux for 3h, distill off dichloroethane and unreacted thionyl chloride. The temperature was lowered below 0°C, and 100 mL of dichloroethane and 45 g of aluminum trichloride were added. Control the temperature below 5°C, slowly feed ethylene from under the liquid surface, and rise to room temperature to react overnight. Then, the reaction solution was poured into ice water containing hydrochloric acid, separated, and the aqueous layer was extracted with dichloroethane. Combine the organic layers, wash with water, wash with 1mol / L sodium hydroxide solution, and wash with water. Put it into a 1L three-necked flask, add 19g of sodium acetate, dropwise add 240mL of 20% peracetic acid, and react for 3d. Pour the...
Embodiment 2
[0053] 1-[N-[N'-[(N"-methoxycarbonyl-N"-phenyl)aminocarbonyl]amino]]imino-5-chloro-2-methoxymethoxy-1,3- Synthesis of Methyl Dihydroinden-2-ylcarboxylate (Compound PC-2)
[0054] In a 20mL three-necked flask, add 100mg of methyl 1-(N-amino)imino-5-chloro-2-methoxymethoxy-1,3-dihydroinden-2-ylcarboxylate, N-chloroform 72 mg of methyl acyl-N-phenylcarbamate, 10 mL of ethyl acetate, 0.66 g of anhydrous potassium carbonate, stirred at room temperature for 15 hours, washed with water, dried, evaporated the solvent, added a small amount of ethanol for recrystallization, and obtained 120 mg of a white solid. The product structure was confirmed to be correct by mass spectrometry and nuclear magnetic resonance spectroscopy.
Embodiment 3
[0056] 1-[N-[N'-[[N"-methoxycarbonyl-N"-(4'-trifluoromethoxy)phenyl]aminocarbonyl]amino]]imino-5-chloro-2-methanol Synthesis of methyl oxymethoxy-1,3-dihydroinden-2-ylcarboxylate (compound PC-3)
[0057] In a 20mL three-necked flask, add 100mg of methyl 1-(N-amino)imino-5-chloro-2-methoxymethoxy-1,3-dihydroinden-2-ylcarboxylate, N-chloroform Acyl-N-(4-trifluoromethoxyphenyl)carbamate 85mg, ethyl acetate 10mL, anhydrous potassium carbonate 0.66g, stirred at room temperature for 15h, washed with water, dried, evaporated the solvent, added a small amount of ethanol to weigh Crystallized to obtain 130mg of white solid. The product structure was confirmed to be correct by mass spectrometry and nuclear magnetic resonance spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com