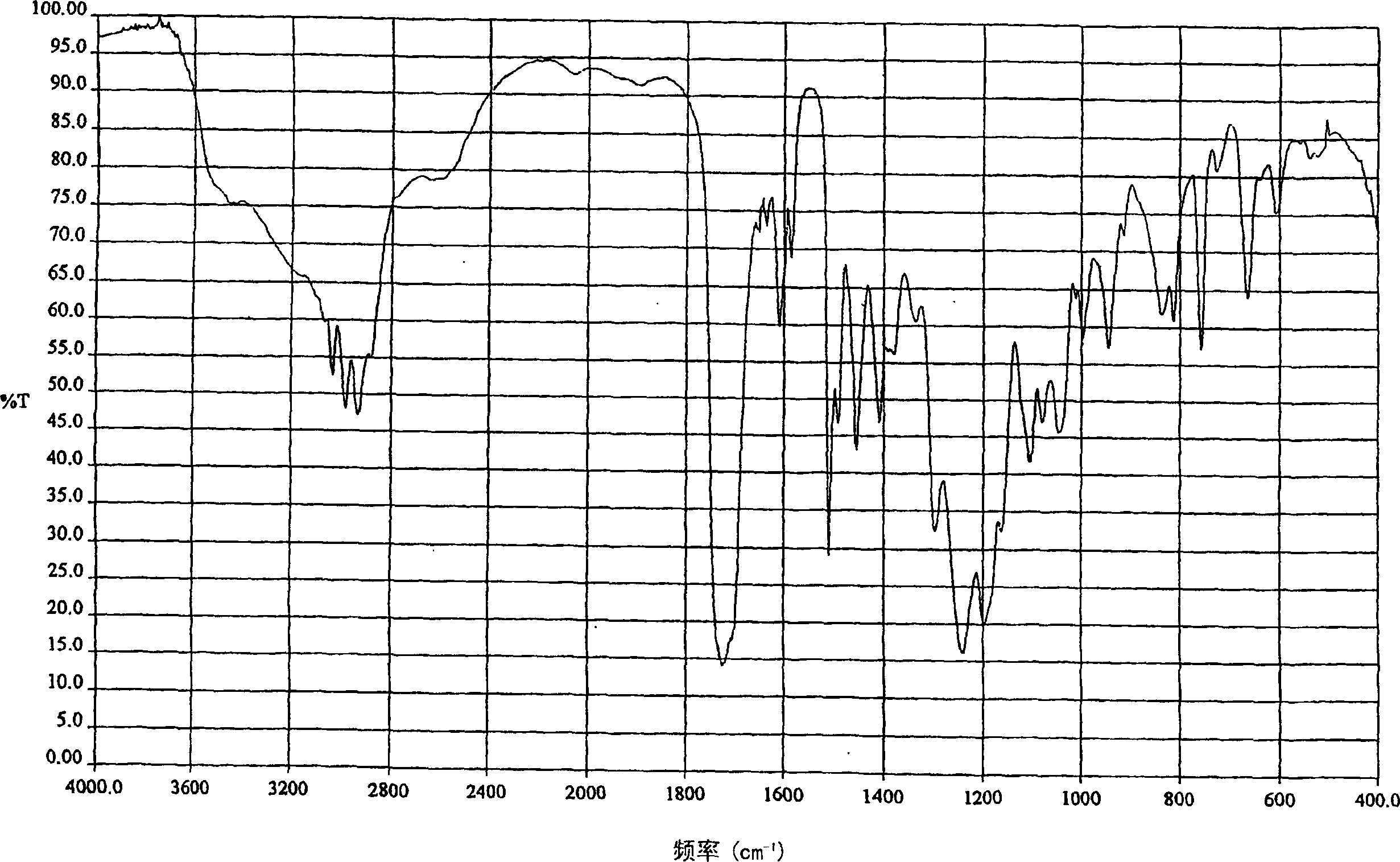
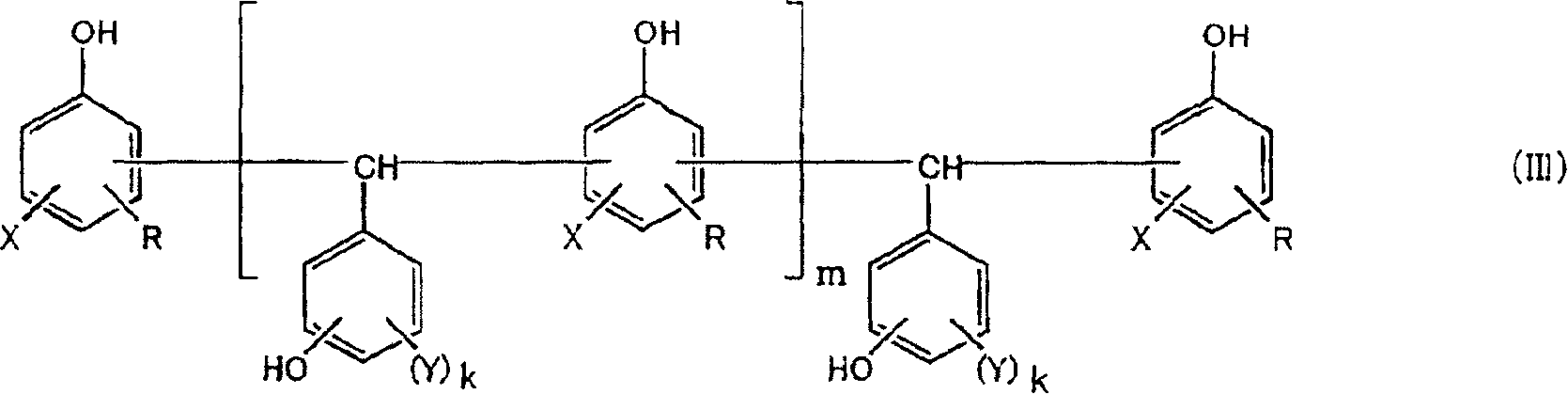
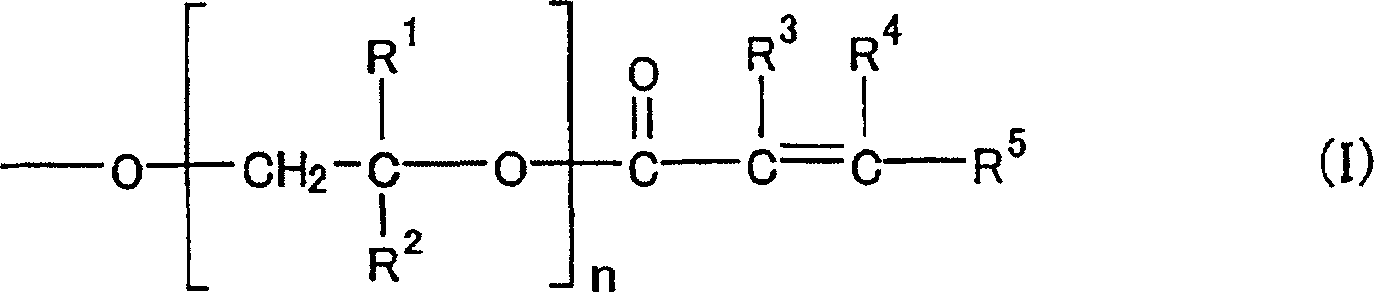
Curable resins and curable resin compositions containing the same
A technology of curable resin and resin composition, applied in the field of curable resin composition, can solve problems such as extremely difficult to achieve practical application, lower heat resistance or moisture resistance, and limit of safe use range, and achieve excellent reactivity, excellent The effect of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0099] In an autoclave equipped with a thermometer, a nitrogen gas introduction device, an alkylene oxide introduction device and a stirring device, a polyphenol resin (manufactured by Japan Epoxy Resin Co., Ltd., trade name: "EPICYUA-YL6065", 98 parts of OH equivalent: 98), 0.98 parts of potassium hydroxide and 98 parts of toluene, the reaction system was replaced with nitrogen under stirring, and the temperature was raised by heating. Subsequently, 63.8 parts of propylene oxide was slowly dropped into it, and at 125 to 132 ° C, 0 to 4.8 kg / cm 2 The reaction was carried out for 16 hours. Thereafter, after cooling to room temperature, 1.28 parts of 89% phosphoric acid was added to the reaction solution, mixed, and neutralized with potassium hydroxide to obtain a polyphenol with a non-volatile content of 63.8% and a hydroxyl equivalent of 160.7 g / eq. Propylene oxide reaction solution of the resin. This is obtained by adding an average of 1.08 moles of epoxy per 1 equivalent o...
Synthetic example 2
[0104] In the autoclave with a thermometer, a nitrogen introduction device, an alkylene oxide introduction device and a stirring device, add 98 parts of polyphenol resin (manufactured by Japan Epoxy Resin Co., Ltd., trade name "EPIQUA-YL6065", OH equivalent: 98), hydrogen 0.98 parts of potassium oxide and 98 parts of toluene were used, and the reaction system was replaced with nitrogen while stirring, and the temperature was raised by heating. Subsequently, 174.0 parts of propylene oxide was slowly dropped into it, and at 125 to 132 ° C, 0 to 4.8 kg / cm 2 The reaction was carried out for 16 hours. Thereafter, after cooling to room temperature, 1.28 parts of 89% phosphoric acid was added to the reaction solution, mixed, and neutralized with potassium hydroxide to obtain a polyphenol with a non-volatile content of 75.1% and a hydroxyl equivalent of 264.5 g / eq. Propylene oxide reaction solution of the resin. This is obtained by adding an average of 2.87 moles of epoxy per 1 equi...
Synthetic example 3
[0108] In an autoclave equipped with a thermometer, a nitrogen gas introduction device, an alkylene oxide introduction device and a stirring device, polyparahydroxystyrene resin (manufactured by Maruzen Petrochemical Co., Ltd., trade name "Maerca-M", OH equivalent: 120) was added. 120 parts, 1.2 parts of potassium hydroxide and 120 parts of toluene, the reaction system was replaced with nitrogen under stirring, and the temperature was raised by heating. Subsequently, 63.8 parts of propylene oxide was slowly dropped into it, at 125 to 132 ° C, 0 to 4.8 kg / cm 2 The reaction was carried out for 16 hours. Thereafter, after cooling to room temperature, 1.57 parts of 89% phosphoric acid was added to the reaction liquid, mixed, and neutralized with potassium hydroxide to obtain poly-p-hydroxyl with 62.0% non-volatile content and 182 g / eq. Propylene oxide reaction solution of styrene resin. This is obtained by adding an average of 1.07 moles of epoxy per 1 equivalent of phenolic hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl equivalent | aaaaa | aaaaa |
| Solid content acid value | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com