Fat ester carbonate polymer with side carboxy, its synthesis and use thereof
A technology of aliphatic carbonate and benzyloxycarbonyl trimethylene carbonate, which is applied in the field of synthesis of aliphatic carbonate monomers and their polymers, which can solve the difficulties of bonding drugs, limited applications, and unsatisfactory biodegradability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
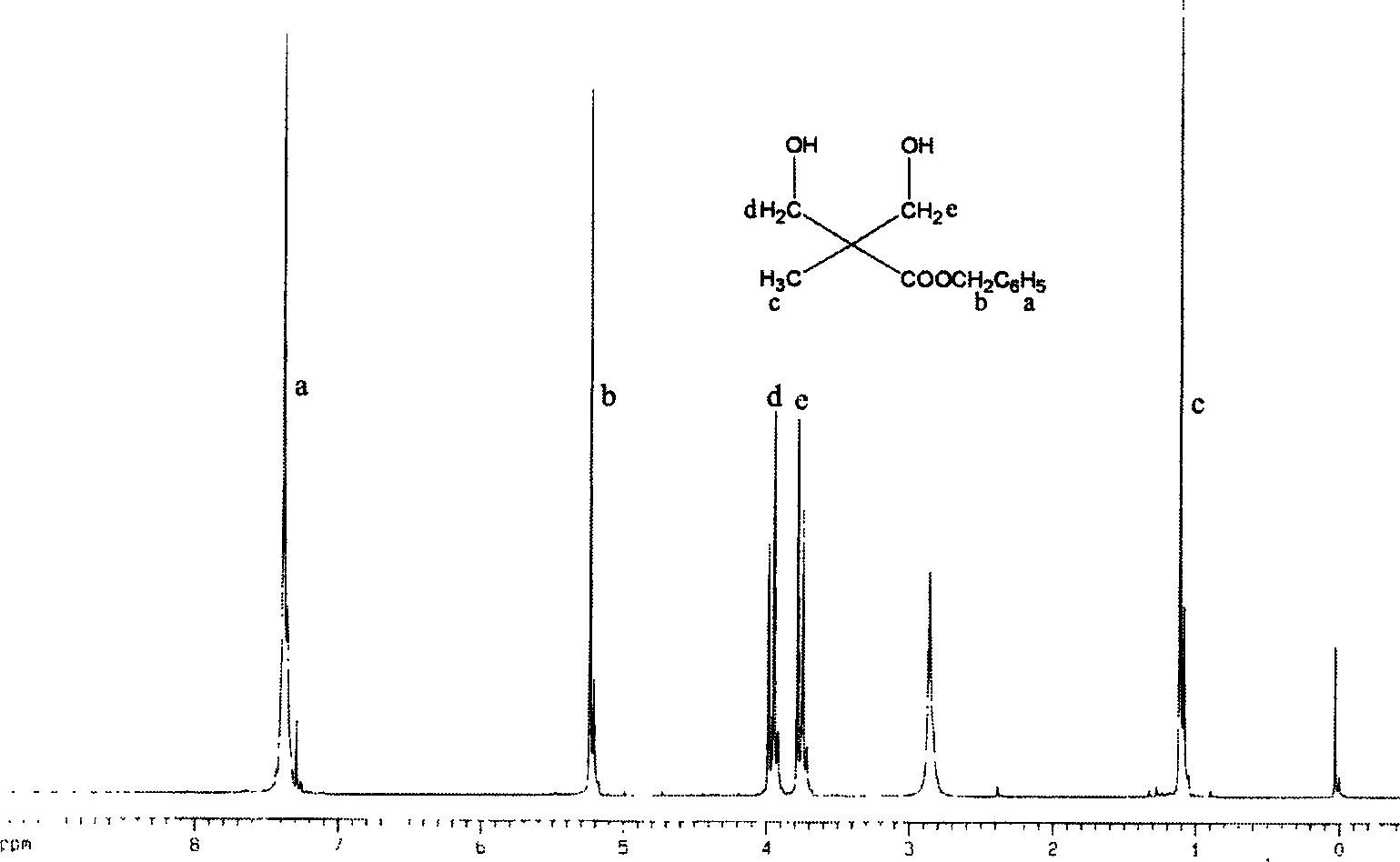
[0027] Embodiment 1: the synthesis of 2-methyl-2-benzyloxycarbonyl-1,3-propanediol
[0028] 9.00 g of 2,2-dimethylolpropionic acid and 4.30 g of potassium hydroxide were dissolved in 50 ml of N,N-dimethylformamide. Stir vigorously for 1 h at 100°C to form potassium salt of 2,2-dimethylolpropionate. Then 13.80 g of benzyl bromide was added to the above solution, and stirred vigorously for another 15 h at 100°C. The solvent was distilled off, the residue was dissolved in 200 ml of ether, washed three times with 50 ml of distilled water, and finally recrystallized with toluene to obtain 8.95 g of 2-methyl-2-benzyloxycarbonyl-1,3-propanediol with a yield of 60%. Its structure was confirmed by proton NMR spectroscopy, see attached figure 1 .
Embodiment 2
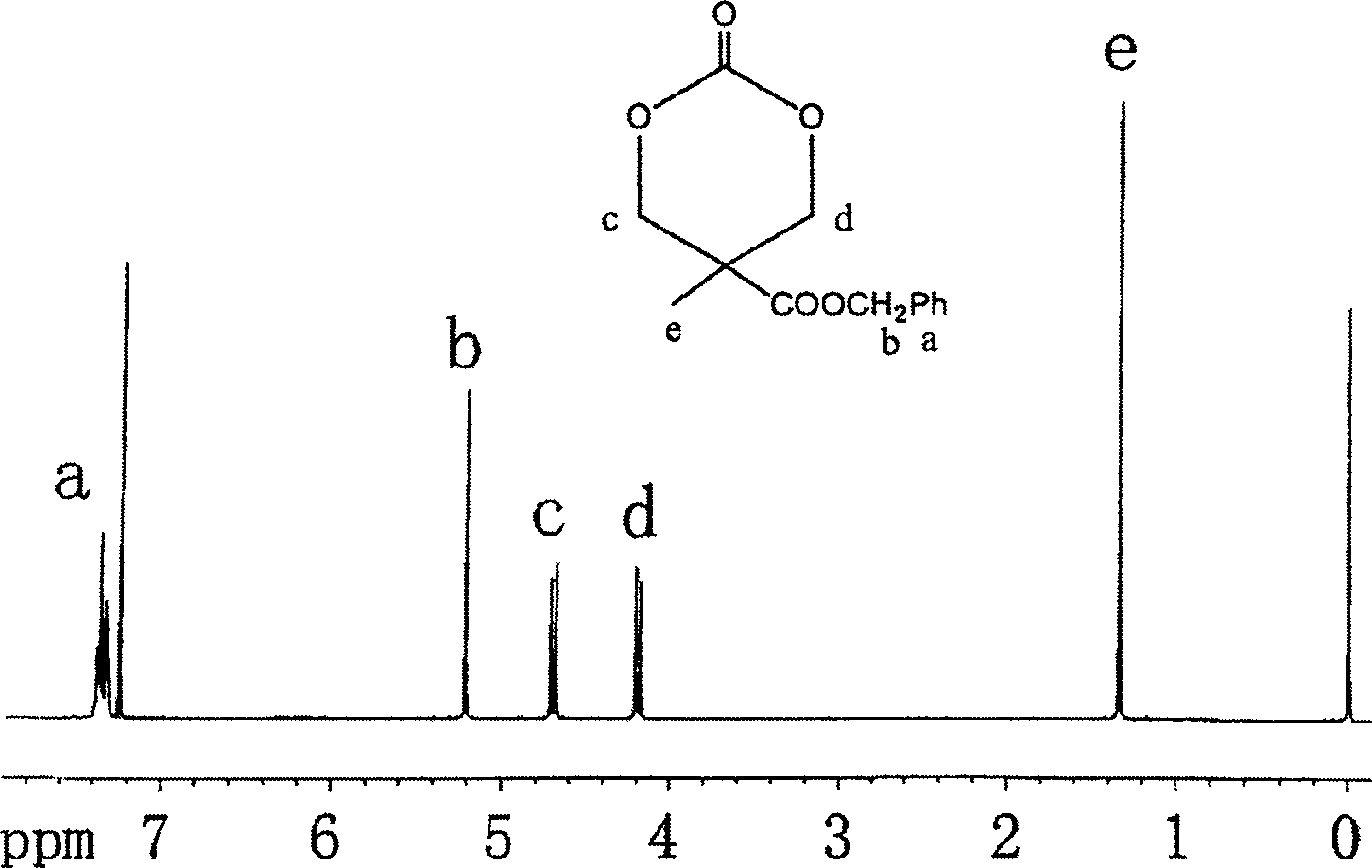
[0029] Embodiment 2: the synthesis of 2-methyl-2-benzyloxycarbonyl trimethylene carbonate
[0030] Dissolve 10 g of 2-methyl-2-benzyloxycarbonyl-1,3-propanediol and 28.5 g of ethyl chloroformate in 600 ml of tetrahydrofuran, and cool in an ice-water bath. Then 28 g of triethylamine was slowly added to the above solution, and the system was kept at 0° C. during the addition. Then react at room temperature for 10h. The precipitate was filtered off, the filtrate was concentrated under reduced pressure, and the residue was recrystallized from tetrahydrofuran and diethyl ether to obtain 9.0 g of white crystals with a yield of 82%. Its structure was confirmed by proton NMR spectroscopy, see attached figure 2 .
Embodiment 3
[0031] Embodiment 3: Synthesis of copolymers of 2-methyl-2-benzyloxycarbonyl trimethylene carbonate and lactic acid in different proportions
[0032] Under anhydrous and oxygen-free conditions, take different proportions of 2-methyl-2-benzyloxycarbonyl trimethylene carbonate and lactide monomer, and add ethyl zinc of 1 / 200 of the total mass of the monomer as a trigger reagent, stirred and reacted at 100°C for 20h, the product was dissolved in dichloromethane, settled with methanol, filtered, washed, dried in vacuo at 35°C for 24h, weighed to calculate the yield, and different proportions of 2-methyl-2-benzyloxy Copolymer of carbonyl trimethylene carbonate and lactic acid. The obtained polymerization results are shown in Table 1.
[0033] Table 1. Copolymers of 2-methyl-2-benzyloxycarbonyl trimethylene carbonate and lactic acid in different proportions
[0034]
[0035] 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com