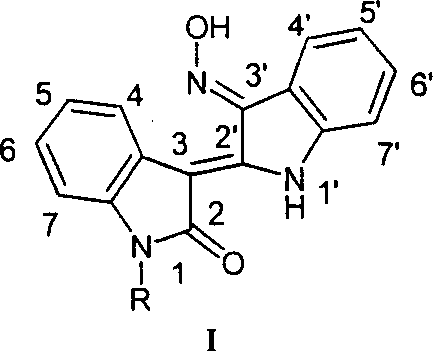
Preparation method and medical uses of Nú¿1ú®-hydrocarbyl-3íõ-nitrotylindirubin derivative 1
A technology of indirubin and derivatives, which is applied to the preparation of N(1)-hydrocarbyl-3′-oxime indirubin derivatives (I) and its medical application field, can solve the problem of poor water solubility and oil solubility , the compound polarity is large, the bioavailability is poor, etc., to achieve the effect of economical method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
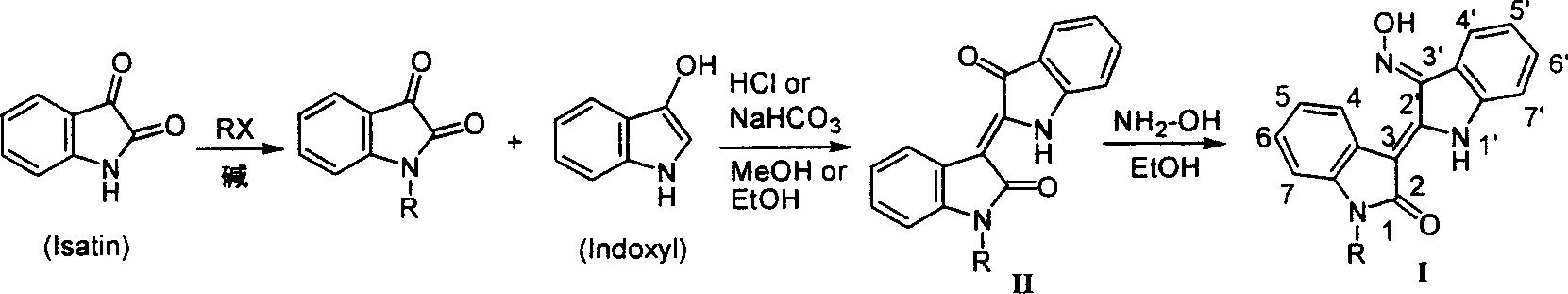
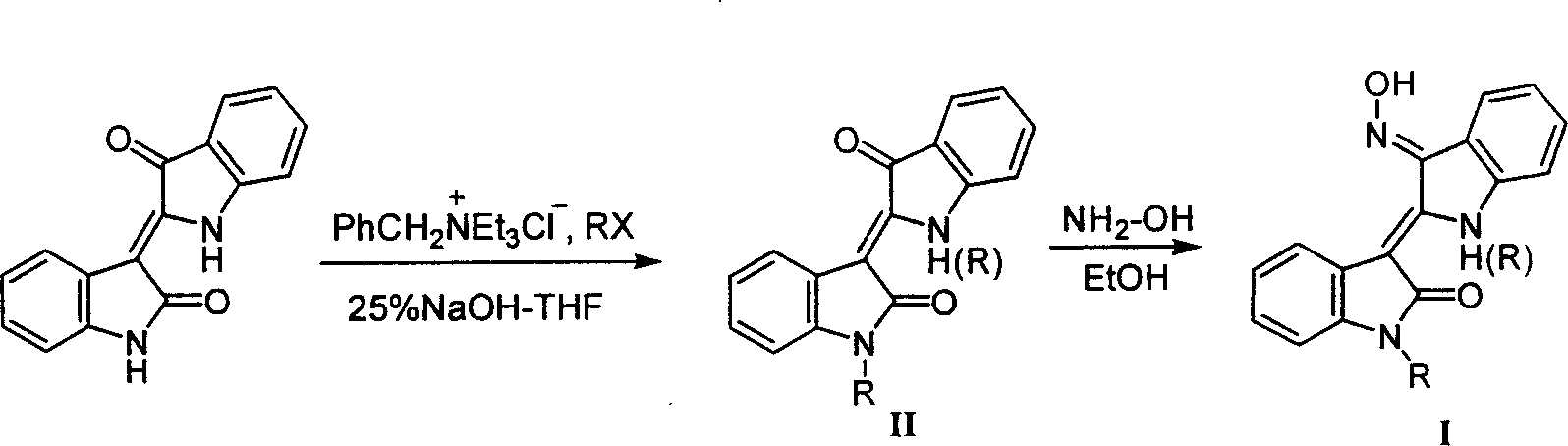
[0062] Experimental Example 1, the preparation of N(1)-hydrocarbyl-3'-oximino indirubin:
[0063] Step a, 1-ethyl-indirubin
[0064] [Step a]: Add 0.6 g of NaH (15 mmol) to a solution consisting of 2.62 g of indirubin (10 mmol) and 40 mL of anhydrous DMF, and 2 Stir under ambient conditions, and cool the mixture to 0°C, slowly add dropwise a solution consisting of 1.15mL ethyl iodide (15mmol) and DMF (5mL), return to room temperature slowly after the addition, stir for 3 hours, and detect by TLC (the developer is the same as above) . After the reaction was completed, carefully poured into 300mL ice water under stirring, extracted 3 times with chloroform (150mL×3), combined the organic phases, and washed with anhydrous Na 2 SO 4 After drying, it was concentrated to dryness in vacuo, and the residue was purified by silica gel column chromatography (eluent: chloroform / petroleum ether, 5 / 1 (v / v)) to obtain 1.5 g of purple powder solid 1-ethyl-indirubin , yield 52%; melting poi...
experiment example 2
[0070] Experimental example 2, the in vitro anti-tumor effect of N(1)-hydrocarbyl-3'-oximino indirubin:
[0071] Inhibitory effects of N(1)-alkyl-3'-oximinoindirubin and N(1)-acetyl-indirubin hormones on dependent and independent prostate cancer.
[0072] The following are some examples of tumor treatment by JN-2528 (N(1)-hydrocarbyl-3'-oximino indirubin) compound. Here again, we reiterate that although we only use the treatment of tumors as an example, it does not mean that these compounds are only used to treat tumors.
[0073] (1) Materials and methods
[0074] Reagent: JN-2528 compound, synthesized and purified by our laboratory, the structure was confirmed by mass spectrometry, NMR, infrared, and X-ray diffraction (see Example 1). The purity was >98.0%. JN-2528 is red and dark red crystalline powder. During the experiment, the solution was prepared in DMSO and stored at -20°C. European patent similar compound Indirubin-3'-monoxime-5 Sulphonic acid (IMSA) [EP1079826B1...
experiment example 3
[0091] Experimental example 3, the in vivo anti-tumor effect of N(1)-hydrocarbyl-3'-oximino indirubin
[0092] (1) Materials and methods:
[0093] Materials: C57BL / 6 mice, all male, 25±2g. Provided by Model Animal Center of Nanjing University. Lewis lung cancer cell line, provided by Nanjing University of Traditional Chinese Medicine. JN-2528 freeze-dried powder, batch number: 050418-1. 1mg per bottle, add 1ml of normal saline to make 1mg / ml, provided by Wuxi Jessie Pharmaceutical Company. Cyclophosphamide, produced by Jiangsu Hengrui Pharmaceutical Co., Ltd., batch number 04081921.
[0094] Instrument: Sartorius BP-310 electronic balance. Ultra clean bench. Glass homogenizer. Several sets of surgical instruments.
[0095] Method: Take Lewis lung cancer tumor block under aseptic operation, remove necrotic tissue, mix several tumor blocks, cut into small pieces, add PBS and grind with a glass tissue homogenizer, put it into a centrifuge tube after grinding, and centrifu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com