Plasmid containing recombinant immunological toxin MIP-1ª‡-DT390 aiming at activated Th1 cell, its preparation method and uses
A technology of immunotoxin and recombinant plasmid, applied in the direction of recombinant DNA technology, drug combination, pharmaceutical formula, etc., can solve the problem of ineffective prevention of disease recurrence, avoid preparation process and difficulty in standardization, relieve clinical symptoms, and have good therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
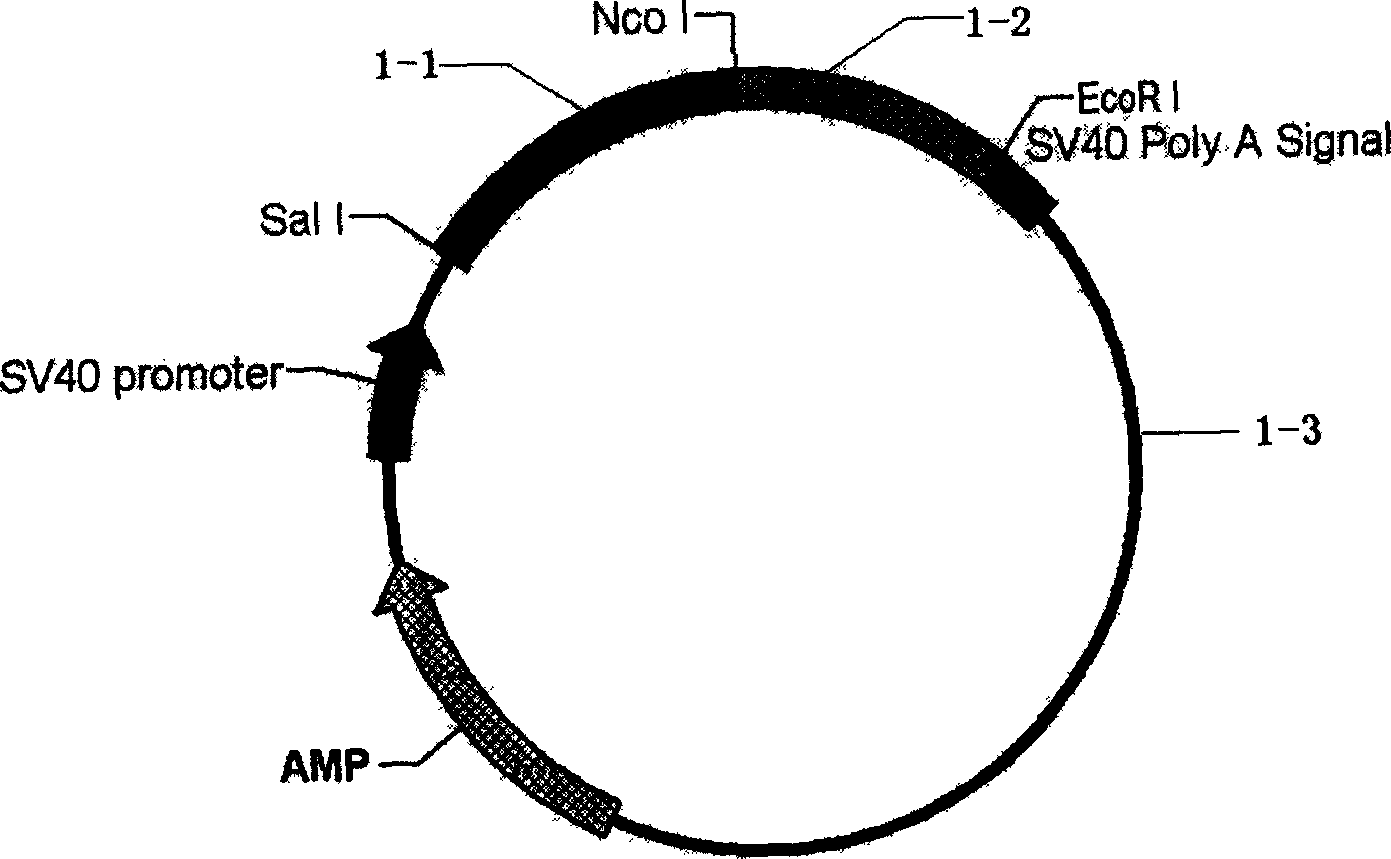
[0030] Example 1: Construction of eukaryotic expression plasmids for recombinant immunotoxins
[0031] The vector is SRα eukaryotic plasmid containing DT390 (provided by PhD. Hu Huaizhong, University of Wisconsin, USA, and can be purchased from Invitrogen Company), and the specific steps are as follows:
[0032] 1. Obtain mouse MIP-1α gene
[0033] (1) Extract mouse liver total RNA with Trizol reagent (purchased from Invitrogen):
[0034] 1) Take 100 mg of mouse liver tissue under sterile conditions.
[0035] 2) Add 1ml Trizol.
[0036] 3) Homogenate (should be thorough, then transfer to EP tubes, when tissue homogenate volume > 100mg, pack separately, 1ml / each EP tube).
[0037] 4) Mix by inverting for 10 times, and let stand at room temperature for 5 minutes.
[0038] 5) Add 1 / 5 volume (0.2ml) of chloroform (must be 1 / 5 of the total volume).
[0039] 6) Mix by inverting for 10 times, and let stand at room temperature for 5 minutes.
[0040] 7) Centrifuge at 12000g for ...
Embodiment 2
[0086] Example 2: Determination of biological activity of recombinant immunotoxin MIP-1α-DT390 in vitro
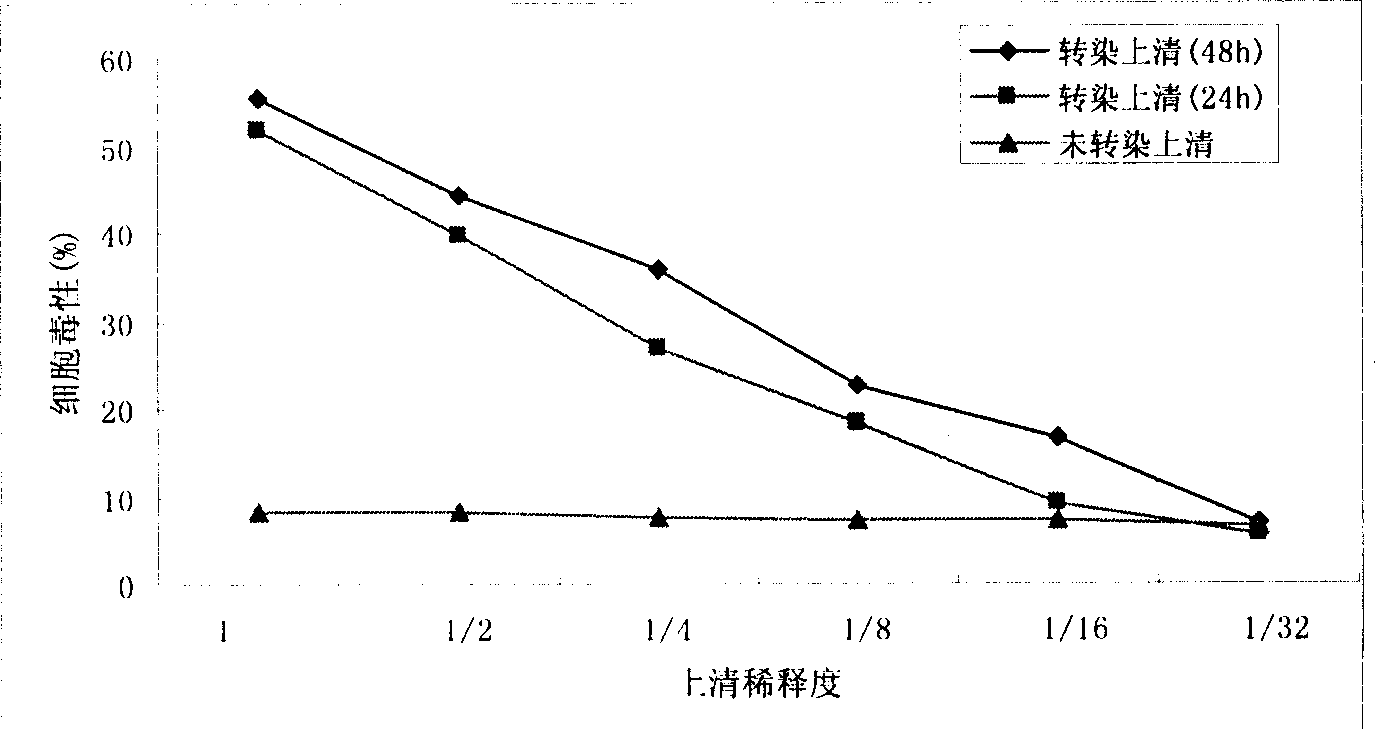
[0087] 1. Cytotoxic effect of MIP-1α-DT390 in transfection supernatant on effector cells (MTT method):
[0088](1) Preparation of effector cells: 6-8 weeks old, about 20g female C57BL / 6 mice were killed after eyeball bleeding, aseptically dissected and stripped the spleen, gently pressed the spleen tissue with a syringe needle, added RPMI1640 medium, collected and centrifuged, Obtain a suspension of splenocytes and adjust the cell concentration to 5 x 10 6 / ml, inoculated in a cell culture flask, added RPMI1640 medium containing concanavalin A (ConA) (final concentration of ConA was 5 μg / ml), cultivated in a 5% CO2 incubator at 37°C, and cultivated for 72 hours Afterwards, ConA-activated lymphocytes grown in suspension were collected as effector cells.
[0089] (2) Adjust the collected effector cell concentration to 1×10 6 / ml, add to 96-well cell culture plate, 100μl / w...
Embodiment 3
[0098] Example 3: Preliminary therapeutic effect of eukaryotic plasmid of recombinant immunotoxin MIP-1α-DT390 on autoimmune disease animal model EAE
[0099] 1. Establishment of EAE animal model: C57BL / 6 mice were immunized with myelin basic protein (MBP) according to a conventional method to establish an EAE model.
[0100] (1) C57BL / 6 mice were used to establish an EAE animal model, and the self-extracted MBP crude extract was mixed with complete Freund adjuvant (full freund adjuvant, FCA) (containing Mycobacterium tuberculosis 5mg / ml) in equal volumes, Use a 3ml syringe to repeatedly push and pull to form a water-in-oil emulsion, and use the method of intraperitoneal injection.
[0101] (2) Immunization dose: each mouse was injected with 0.4ml of MBP:FCA (1:1) mixture, 0.2ml of pertussis bacterial liquid:PBS (1:50) mixture (containing 0.6-1.8×10 6 indivual).
[0102] (3) In the control group, each mouse was intraperitoneally injected with 0.2ml of a mixture of Bacillus p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com