1,2,4-substituerte 1,2,3,4-tetrahydro-and 1,2 dihydro-quinoline and 1,2,3,4-tetrahydro-quinoxaline derivatives as cetp inhibitors for the treatment of atherosclerosis and obesity
A three-substitution and two-substitution technology, which is applied to the active ingredients of heterocyclic compounds, anti-toxic agents, anti-inflammatory agents, etc., can solve the problems of not meeting medical needs, increasing plasma HDL levels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
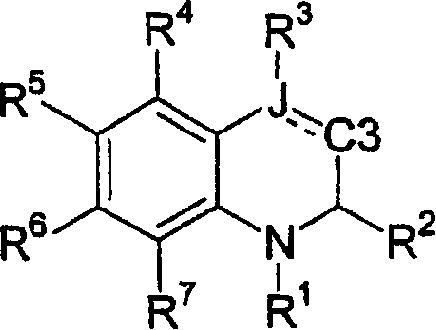
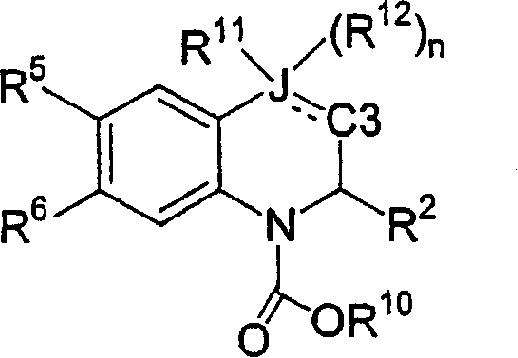
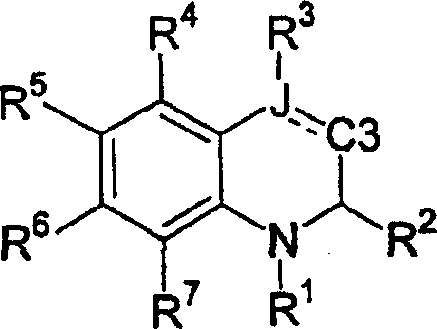
[0301] An alternative method of preparing the desired compound of formula II is from the corresponding compound of formula X by using a chlorinating reagent such as phosphorus(III) chloride or thionyl chloride in a reaction inert solvent such as dichloromethane or chloroform optionally This is achieved by treatment comprising a base such as pyridine, diisopropylethylamine or 2,6-di-tert-butyl-4-methylpyridine at 0°C to 60°C, usually ambient temperature, for 1 to 24 hours, wherein L is (C 1 -C 6 ) alkoxycarbonyl and V, R 1 , R 2 , R 4 , R 5 , R 6 and R 7 as defined above. The non-parasitic formed is then treated with a fine metal powder such as zinc in the presence of an acid or mixed acids such as acetic acid or hydrochloric acid in a suitable solvent or mixed solvents such as methanol, water or tetrahydrofuran at 25°C to 60°C, usually ambient temperature. Chlorine-containing derivatives as a mixture of enantiomers afford the desired products of formula II.
[0302] T...
Embodiment 1
[0589]
[0590] 4-(3,5-Di-trifluoromethyl-benzoyl)-6,7-dimethoxy-2-methyl-2H-quinoline-1-carboxylic acid ethyl ester
[0591] Ethyl 4-diazo-6,7-dimethoxy-2-methyl-3,4-dihydro-2H-quinoline-1-carboxylate (Preparation 7, 0.65 mmol) prepared as above Diethyl ether solution was added to 3,5-bistrifluoromethylbenzoyl chloride (180 mg, 0.65 mmol) and N, N-diisopropylethylamine (120 μl, 0.65 mmol) in diethyl ether solution (15 mL) and heated at room temperature Stir in the dark under nitrogen for 15 hours. The solvent was removed in vacuo and the residue was chromatographed on silica gel eluting with 3:1 hexane:ethyl acetate to afford partially purified product which was subjected to reverse phase chromatography (linear acetonitrile:water gradient, 55% to 100 % acetonitrile, both phases contained 0.1% formic acid) to give the title compound as a lemon yellow solid (90 mg).
[0592] MS: 518.1[M+H] + measured value
[0593] 1 H-NMR (CDCl 3 ): δ8.26(s, 2H), 8.07(s, 1H), 7.25(brs...
Embodiment 2
[0595]
[0596] (R)-4-[(3,5-Di-trifluoromethyl-phenyl)-hydroxy-methoxycarbonyl-methyl]-2-ethyl-6-trifluoromethyl-2H-quinoline -1-Carboxylic acid ethyl ester
[0597] A solution of 3,5-bis(trifluoromethyl)benzene bromide (0.818 mL, 4.74 mmol) in THF (0.7 mL) was added dropwise to stirred magnesium powder (116 mg, 4.74 mmol) in anhydrous THF ( 4.7 mL) solution to prepare 3,5-bis(trifluoromethylphenyl)magnesium bromide solution. The mixture was then heated to reflux for 1 hour to give a dark solution. A portion of this solution (1.5 mL) was added dropwise at 78 °C to ethyl (R)-2-ethyl-4-methoxycarbonyl-6-trifluoromethyl-2H-quinoline-1-carboxylate (Preparation 10, 133mg, 0.345mmol) in anhydrous THF solution (4mL). After 30 minutes, the mixture was warmed to 0°C and poured into water, extracted with ethyl acetate, and a few drops of 2N hydrochloric acid were added to facilitate separation of the layers. The organic layer was dried over anhydrous sodium sulfate and evaporated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com