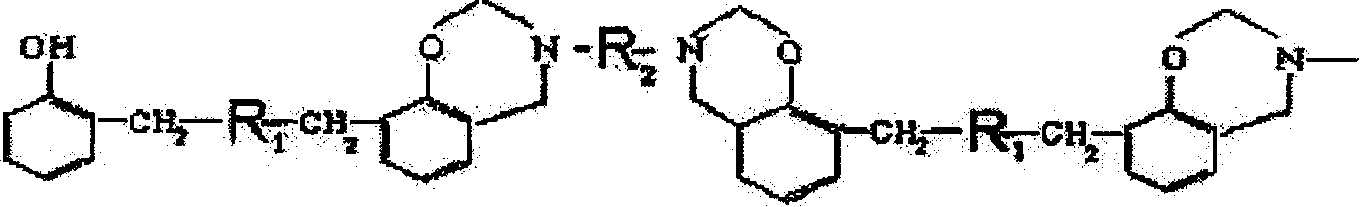
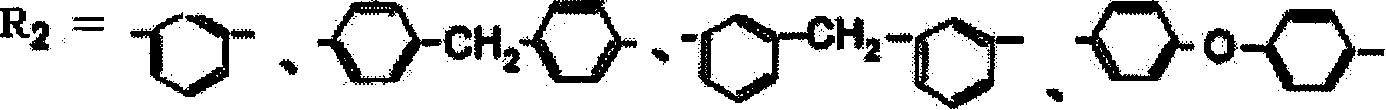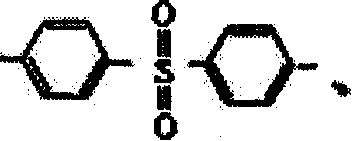Benzoxazine resin containing aralkyl structure, its preparation method and use
A technology of aralkyl and benzoxazine, which is applied in the field of benzoxazine resin and its preparation, can solve the problems of large void ratio, long post-processing time, low retention rate of thermal mechanical strength, etc., and achieve synthetic reaction Smooth, easy to operate, easy to achieve the effect of industrialized continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Add 100 parts of methoxyxylene monomer, 120 parts of phenol and 2 parts of p-toluenesulfonic acid into the reaction kettle, heat up (not exceeding 140°C) for dehydration, and lower the temperature when the released water reaches a certain amount After cooling, add 360 parts of formaldehyde, use 8 parts of basic catalyst to adjust the pH value of the system to 6-10, add toluene, 120 parts of 4,4'diaminodiphenylmethane (MDA) and carry out temperature-raising reflux reaction for 6 hours, then The benzoxazine resin containing aralkyl structure can be obtained by vacuum dehydration at 80-140°C; the solvent is added to obtain a stable solution of benzoxazine resin containing aralkyl structure that is uniform, transparent, and brown-red. The resin solution can be added with an accelerator according to different uses to adjust the molding time to 1-8min / 160°C.
Embodiment 2
[0062] Example 2: Add 80 parts of methoxydiphenyl ether monomer, 80 parts of phenol and 1 part of p-hydrochloric acid into the reaction kettle, heat up (not exceeding 140°C) for dehydration, and cool down when the released water reaches a certain amount, Then add 160 parts of formaldehyde, use 6 parts of basic catalyst to adjust the pH value of the system to 6-10, add toluene, 40 parts of ethylenediamine to carry out temperature-raising and reflux reaction for 6 hours, and vacuum dehydrate at 80-140 ° C to obtain aromatic The benzoxazine resin with an alkyl structure is added with a solvent to obtain a stable solution of the benzoxazine resin with an aralkyl structure that is uniform, transparent, and brownish red. The resin solution can be added with an accelerator according to different uses to adjust the molding time to 1-8min / 160°C.
Embodiment 3
[0063] Example 3: Add 100 parts of methoxydiphenyl ether monomer, 80 parts of phenol and 3 parts of p-toluenesulfonic acid into the reaction kettle, heat up (not exceeding 140°C) for dehydration, and lower the temperature when the released water reaches a certain amount Cool, then add 160 parts of formaldehyde, use 4 parts of basic catalyst to adjust the pH value of the system to 6-10, add toluene, 40 parts of ethylenediamine, carry out temperature rise and reflux reaction for 4 hours, then vacuum dehydrate at 80-140 ° C to obtain The benzoxazine resin containing an aralkyl structure is added with a solvent to obtain a stable solution of the benzoxazine resin containing an aralkyl structure that is uniform, transparent, and brownish red. The resin solution can be added with an accelerator according to different uses to adjust the molding time to 1-8min / 160°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com