Novel tyrosinase inhibitor and use thereof
A technology of tyrosinase and inhibitors, applied in the field of biphenyl glycoside compounds, can solve problems such as difficult to maintain stable product quality, achieve good skin pigmentation, improve melanin production inhibition effect and whitening effect, and prevent skin pigmentation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
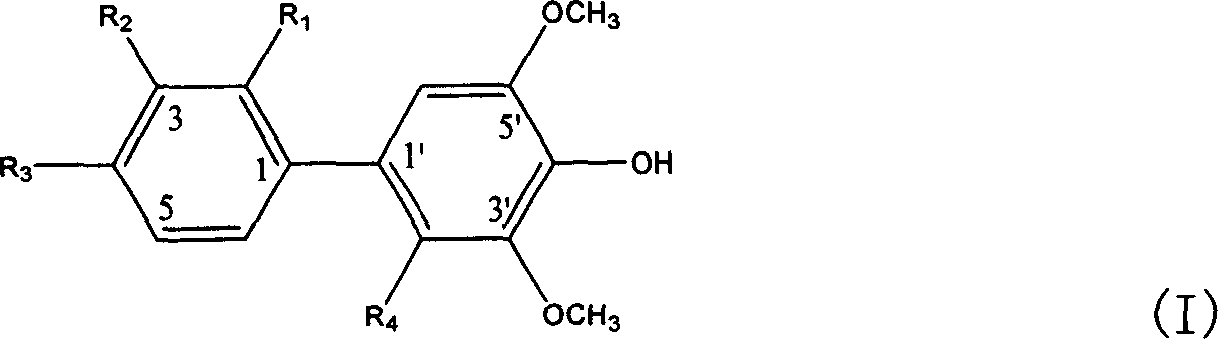
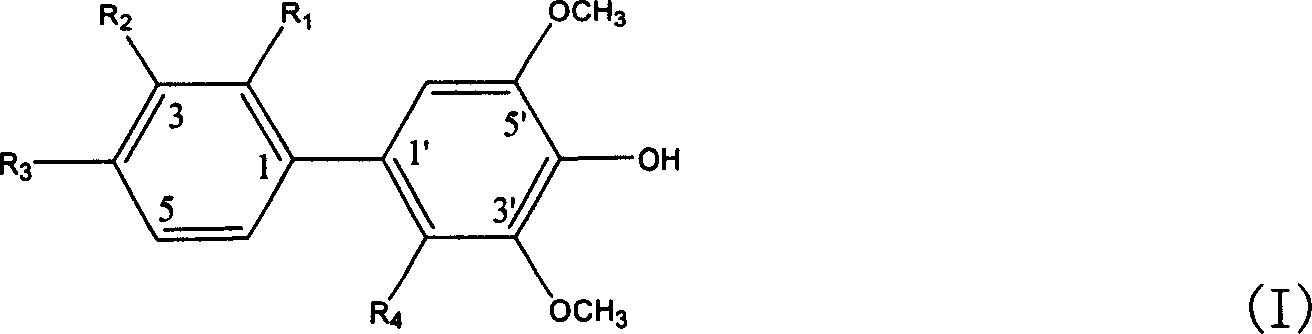
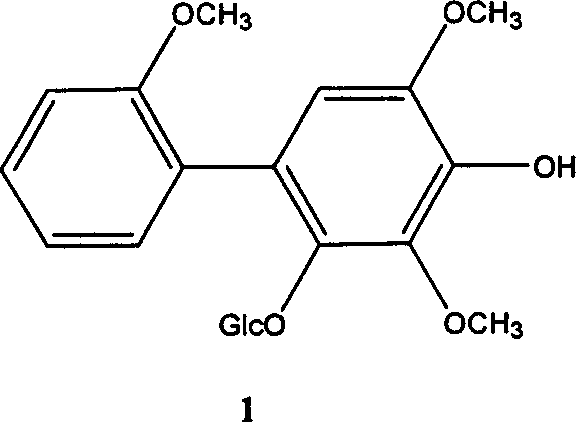
[0027] Embodiment 1, the preparation of the biphenyl glycoside derivative represented by the following formula I
[0028]
[0029] Take the dried fruit of Pyracantha fortuneana in Qinling Mountains, Shaanxi, use 5 times the amount of 60% ethanol solution, heat reflux to extract three times, each time for 2 hours, combine the extracts, filter to remove insoluble matter, and concentrate the filtrate under reduced pressure to dry. The compound was dispersed in an equal volume of water and extracted with chloroform and n-butanol successively. The n-butanol extraction part was dried under reduced pressure in vacuum to obtain the n-butanol extract. Subsequently, carry out chromatography with macroporous adsorbent resin open column, be respectively 10, 30, 50, 70, 95% ethanol aqueous solution gradient elution with concentration; Get 30% ethanol aqueous solution elution part and carry out silica gel open column chromatography, The chloroform:methanol was 20:1, 10:1, 4:1, 2:1, 1:1 ...
Embodiment 2
[0048] Example 2: Determination of Tyrosinase Inhibitory Activity
[0049] Test samples: Compounds 1-4 prepared in Example 1 were taken, and tyrosinase was purchased from Sigma Company and extracted from mushrooms.
[0050] Reference substance: arbutin
[0051] Test method: firstly, the substrate L-tyrosine was dissolved in a phosphate buffer solution (25 mM, pH 6.8) to reach a concentration of 0.1 mg / mL. Into a 96-well plate, 40 μL of the above substrate solution, 80 μL of phosphate buffer (25 mM, pH 6.8), and 40 μL of formula (I) dissolved in 20% methanol-water were added. Add 40 μL of 20% methanol-water to the blank control air. Add 40 μL of tyrosinase in phosphate buffer solution (100 U / mL, 25 mM, pH 6.8) to start the reaction, and incubate at 37° C. for 30 minutes. The absorbance of each well was measured at 492 nm before and after incubation.
[0052] According to the absorbance at 492nm, the inhibitory rate (%) of compound (I) to tyrosinase is calculated, and the co...
Embodiment 3
[0061] Example 3: Whitening Cream
[0062] Prescription: compound 3 0.1g cysteine 0.1g
[0063] Stearic acid 10.0g Cetyl alcohol 4.5g
[0064] Lanolin 1.0g Propylene Glycol 15.0g
[0065] Triethanolamine 0.7g Sodium Lauryl Sulfate 0.5g
[0066] Ethyl p-hydroxybenzoate 0.1g Distilled water 68.ml
[0067] Preparation method: mix stearic acid, cetyl alcohol, and lanolin; mix another compound, propylene glycol, triethanolamine, sodium lauryl sulfate, ethyl p-hydroxybenzoate, and distilled water, and heat them in a beaker until melted Or dissolve, store at about 70°C, add the oil phase to the water phase with stirring, and stir until condensed to obtain
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com