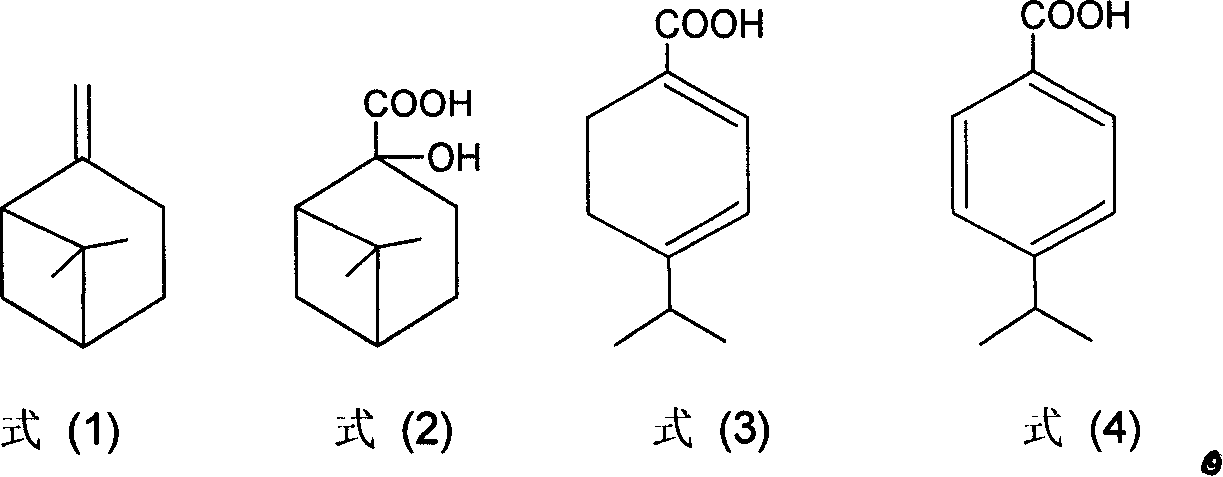
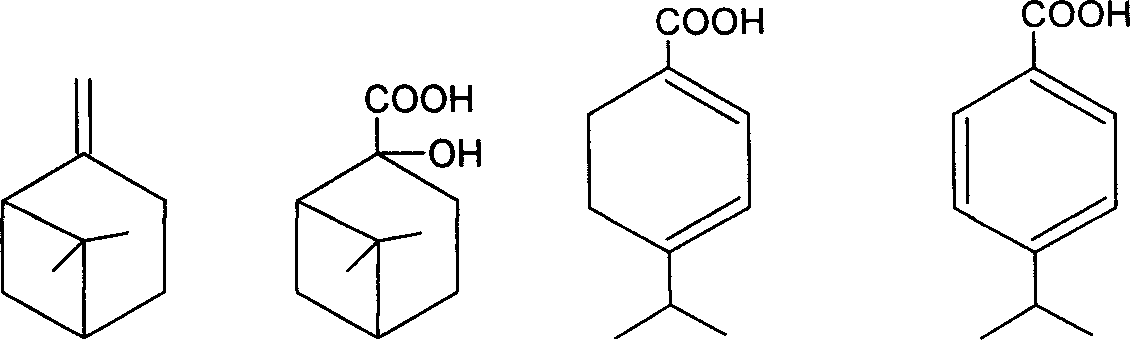
New method for synthesizing p-isopropyl benzoic acid
A technology of isopropyl benzoic acid and a new method, which is applied in the field of synthesizing p-isopropyl benzoic acid, can solve problems such as difficult recovery of acetic anhydride, few deep-processed products, and high price of acetic anhydride, and achieve abundant raw materials, low cost, and low price. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 4g of sodium hydroxide and 45g of potassium permanganate in 450mL of water, and add 13.6g of β-pinene in batches under vigorous stirring. Use a water bath to maintain the reaction temperature at 25-30°C, react for 3-5 hours, and finish the reaction with potassium permanganate as the end point. The precipitated manganese dioxide was filtered off and washed several times with water. The filtrate was concentrated to 150 mL by evaporation under reduced pressure, and left overnight in the refrigerator. Filtrate under reduced pressure and wash with a small amount of ice water to obtain white norpinate sodium crystals.
[0020] Add the sodium pinoleate obtained above into a mixture of 20 mL of water and 25 mL of dichloromethane, adjust to strong acidity with 6 mol / L hydrochloric acid solution, separate the dichloromethane, wash the aqueous phase three times with 7 mL of dichloromethane, combine the organic phases, Add 5 g of anhydrous sodium sulfate to dry for 1 h, ...
Embodiment 2
[0024] Dissolve 6g of sodium hydroxide and 45g of potassium permanganate in 350mL of water, and add 13.6g of β-pinene in batches under vigorous stirring. Use a water bath to maintain the reaction temperature at 25-30°C, react for 3-5 hours, and finish the reaction with potassium permanganate as the end point. The precipitated manganese dioxide was filtered off and washed several times with water. The filtrate was concentrated to 150 mL by evaporation under reduced pressure, and left overnight in the refrigerator. Filtrate under reduced pressure and wash with a small amount of ice water to obtain 6.5 g of white norpinate sodium crystals.
[0025] Add 10g of norpinate sodium to 200mL 20% sulfuric acid aqueous solution to reflux for 2h, cool and filter to obtain 7g of dihydrocuminic acid.
[0026] Put 80g of dihydrocuminic acid, 3.0g of 7.5% Pd-C catalyst, and 150mL of p-cymene into a 250mL three-necked flask equipped with a thermometer, a stirring magnet, and a condenser tube....
Embodiment 3
[0028] The melting point, hydrogen spectrum and mass spectrum data of the product of embodiment 1,2 are as follows:
[0029] Melting point: 117~119℃
[0030] Hydrogen spectrum: 1 H NMR (CDCl 3 ): δ=1.26(d, 6H, J=6.9Hz); 2.97(m, 1H, J=6.9Hz); 7.30(d, 2H, J=8.2Hz); 8.02(d, 2H, J=8.2Hz ).
[0031] Mass spectrum: MS (m / z): 164 (M + , 42.0), 149(100), 131(22.4), 119(51.8), 105(47.2), 91(18.7), 77(28.2), 65(4.8), 51(7.4), 39(8.0), 27 (5.0).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com