Nanometer solid taxol lipoid particle and its prepn process
A technology of solid lipid nanometer and paclitaxel, which is applied in the directions of liposome delivery, drug combination, pharmaceutical formulation, etc., can solve problems such as poor water solubility, and achieve the effects of preventing hydrolysis and oxidation, increasing compliance, and ensuring stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
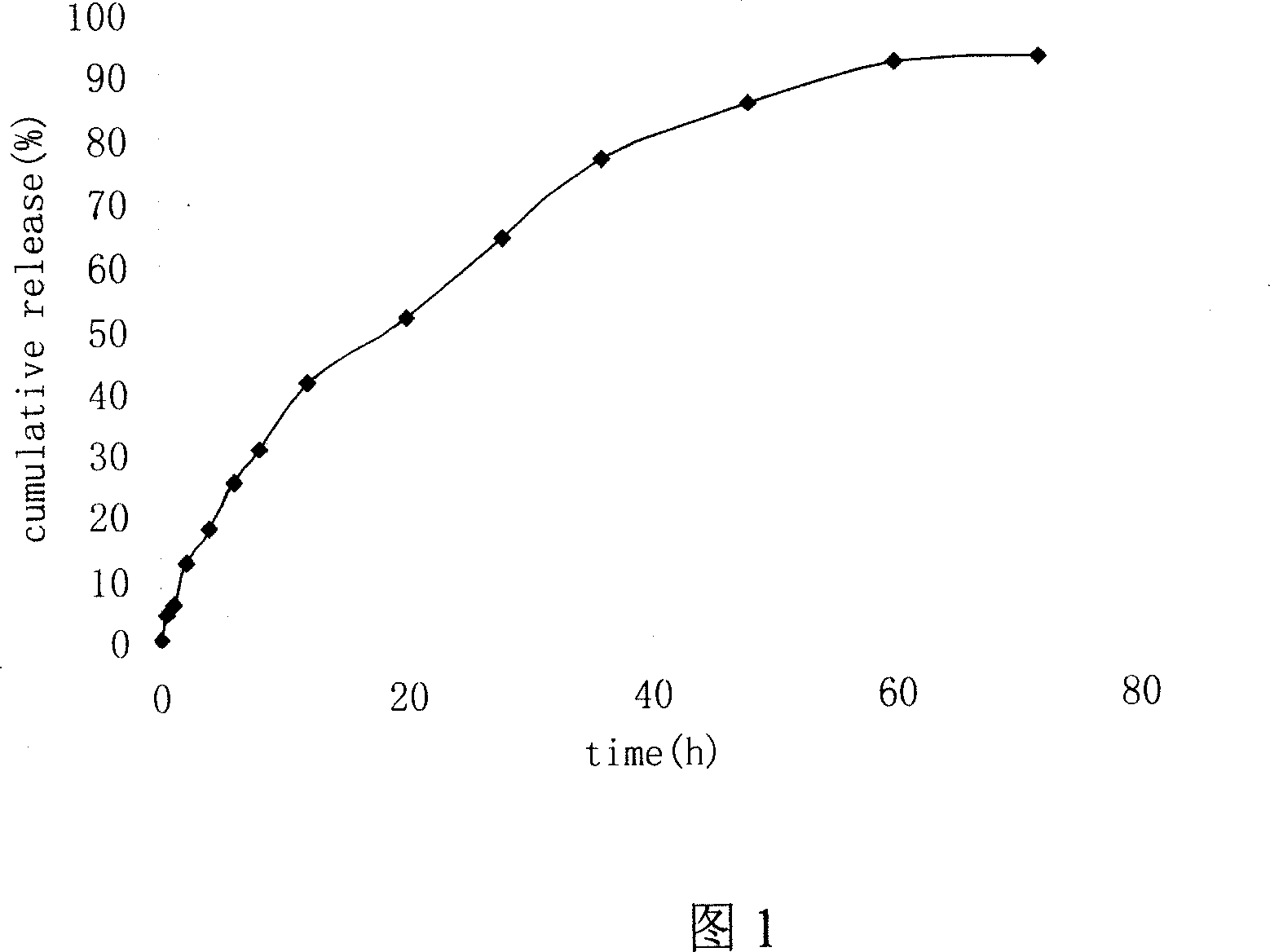
[0036]Take by weighing 20mg paclitaxel, 100mg stearic acid and 100mg lecithin and dissolve in 20ml of methylene chloride, evaporate to dryness under reduced pressure on a rotary evaporator, form a lipid film, add 30ml of Tween-80 (2.5%), and ultrasonically Disperse to obtain the SLN suspension system, and store it at 2°C for later use. Its average particle diameter is 80nm, accounts for 80%, and all particles are all below 150nm, and particle size distribution is narrow, shows that solid lipid nanoparticle size is comparatively uniform; Stable for 12 months, no precipitation and degradation of paclitaxel substances were observed during storage.
Embodiment 2
[0038] Weigh 15mg of docetaxel, 60mg of stearic acid, and 120mg of soybean lecithin into a 50ml Erlenmeyer flask with a stopper, add 10ml of acetone, and ultrasonically dissolve it fully to form an organic phase. Another 200mg of Poloxamer 188 was dissolved in 30ml of double distilled water to form the water phase. Use a syringe to slowly inject the organic phase into the 75°C aqueous phase stirred at 1000r·min, and continue stirring until a translucent system is formed. The organic solvent was removed by evaporation under reduced pressure, and the temperature was lowered to room temperature to obtain a solid lipid nanoparticle suspension.
Embodiment 3
[0040] Take 20 mg of paclitaxel, 100 mg of stearic acid, 400 mg of lecithin and 10 ml of methylene chloride, add them into a 25 ml pear-shaped bottle with a stopper, and ultrasonically dissolve to form an organic phase. Another 300 mg of polyoxyethylene fatty acid ester Myrj53 was added into 30 ml of double-distilled water, and ultrasonically dissolved to form a water phase. Slowly inject the organic phase into the water phase at a constant temperature of (75±2)°C stirred at 1000r / min to form a microemulsion, and continue stirring for about 2-3 hours to completely evaporate the organic solvent and concentrate the system to about 5ml. Quickly mix the obtained translucent microemulsion into 10 ml of ice-water phase stirred at 1000 r / min at -0 to 2°C, and continue to stir for 2 hours to obtain solid lipid nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com